T cells and reactive oxygen species
- PMID: 26471060
- PMCID: PMC4608155
- DOI: 10.1186/s12929-015-0194-3
T cells and reactive oxygen species
Abstract
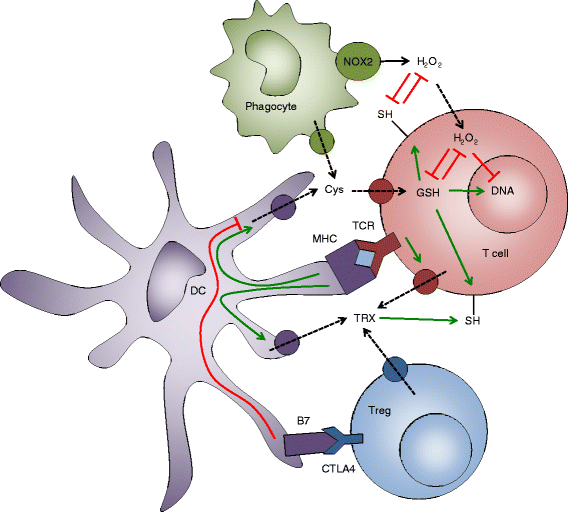
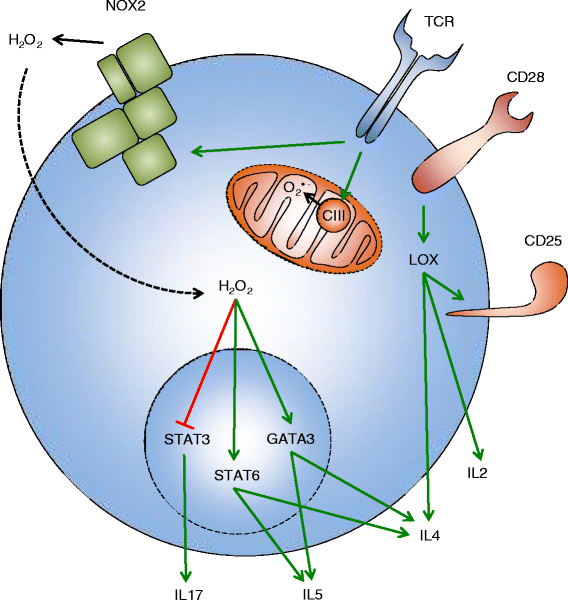
Reactive oxygen species (ROS) have been long considered simply as harmful by-products of metabolism, which damage cellular proteins, lipids, and nucleic acids. ROS are also known as a weapon of phagocytes, employed against pathogens invading the host. However, during the last decade, an understanding has emerged that ROS also have important roles as signaling messengers in a multitude of pathways, in all cells, tissues, and organs. T lymphocytes are the key players of the adaptive immune response, which both coordinate other immune cells and destroy malignant and virus-infected cells. ROS have been extensively implicated in T-cell hyporesponsiveness, apoptosis, and activation. It has also become evident that the source, the kinetics, and the localization of ROS production all influence cell responses. Thus, the characterization of the precise mechanisms by which ROS are involved in the regulation of T-cell functions is important for our understanding of the immune response and for the development of new therapeutic treatments against immune-mediated diseases. This review summarizes the 30-year-long history of research on ROS in T lymphocytes, with the emphasis on the physiological roles of ROS.
Figures

Similar articles
-
Activation of cytotoxic lymphocytes by interferon-alpha: role of oxygen radical-producing mononuclear phagocytes.J Leukoc Biol. 2004 Dec;76(6):1207-13. doi: 10.1189/jlb.0204113. Epub 2004 Sep 10. J Leukoc Biol. 2004. PMID: 15361542
-
T cell receptor stimulation, reactive oxygen species, and cell signaling.Free Radic Biol Med. 2004 Oct 15;37(8):1144-51. doi: 10.1016/j.freeradbiomed.2004.05.029. Free Radic Biol Med. 2004. PMID: 15451054 Review.
-
CTLA-4IG suppresses reactive oxygen species by preventing synovial adherent cell-induced inactivation of Rap1, a Ras family GTPASE mediator of oxidative stress in rheumatoid arthritis T cells.Arthritis Rheum. 2006 Oct;54(10):3135-43. doi: 10.1002/art.22139. Arthritis Rheum. 2006. PMID: 17009234
-
Biological and physiological role of reactive oxygen species--the good, the bad and the ugly.Acta Physiol (Oxf). 2015 Jul;214(3):329-48. doi: 10.1111/apha.12515. Epub 2015 May 29. Acta Physiol (Oxf). 2015. PMID: 25912260 Review.
-
Reactive Oxygen Species Regulate T Cell Immune Response in the Tumor Microenvironment.Oxid Med Cell Longev. 2016;2016:1580967. doi: 10.1155/2016/1580967. Epub 2016 Jul 28. Oxid Med Cell Longev. 2016. PMID: 27547291 Free PMC article. Review.
Cited by
-
Hepatitis B Virus X Protein Induces Reactive Oxygen Species Generation via Activation of p53 in Human Hepatoma Cells.Biomolecules. 2024 Sep 24;14(10):1201. doi: 10.3390/biom14101201. Biomolecules. 2024. PMID: 39456134 Free PMC article.
-
Metabolic regulation of the immune system in health and diseases: mechanisms and interventions.Signal Transduct Target Ther. 2024 Oct 9;9(1):268. doi: 10.1038/s41392-024-01954-6. Signal Transduct Target Ther. 2024. PMID: 39379377 Free PMC article. Review.
-
Gut redox and microbiome: charting the roadmap to T-cell regulation.Front Immunol. 2024 Aug 21;15:1387903. doi: 10.3389/fimmu.2024.1387903. eCollection 2024. Front Immunol. 2024. PMID: 39234241 Free PMC article. Review.
-
The relationship of redox signaling with the risk for atherosclerosis.Front Pharmacol. 2024 Aug 1;15:1430293. doi: 10.3389/fphar.2024.1430293. eCollection 2024. Front Pharmacol. 2024. PMID: 39148537 Free PMC article. Review.
-
Rv0687 a Putative Short-Chain Dehydrogenase Is Required for In Vitro and In Vivo Survival of Mycobacterium tuberculosis.Int J Mol Sci. 2024 Jul 18;25(14):7862. doi: 10.3390/ijms25147862. Int J Mol Sci. 2024. PMID: 39063103 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

