Kaposi's Sarcoma-Associated Herpesvirus Reduces Cellular Myeloid Differentiation Primary-Response Gene 88 (MyD88) Expression via Modulation of Its RNA
- PMID: 26468534
- PMCID: PMC4702530
- DOI: 10.1128/JVI.02342-15
Kaposi's Sarcoma-Associated Herpesvirus Reduces Cellular Myeloid Differentiation Primary-Response Gene 88 (MyD88) Expression via Modulation of Its RNA
Abstract
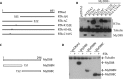
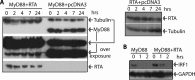
Kaposi's sarcoma (KS)-associated herpesvirus (KSHV) is a human gammaherpesvirus associated with several human malignancies. The replication and transcription activator (RTA) is necessary and sufficient for the switch from KSHV latency to lytic replication. Interleukin 1 (IL-1) is a major mediator for inflammation and plays an important role in both innate and adaptive immunity. Myeloid differentiation primary response gene 88 (MyD88) is an essential adaptor molecule for IL-1 as well as most Toll-like receptor signaling. In this study, we identified a novel mechanism by which KSHV interferes with host inflammation and immunity. KSHV RTA specifically reduces the steady-state protein levels of MyD88, and physiological levels of MyD88 are downregulated during KSHV lytic replication when RTA is expressed. The N-terminal region of RTA is required for the reduction of MyD88. Additional studies demonstrated that RTA targets MyD88 expression at the RNA level, inhibits RNA synthesis of MyD88, and may bind MyD88 RNA. Finally, RTA inhibits IL-1-mediated activation of NF-κB. Because IL-1 is abundant in the KS microenvironment and inhibits KSHV replication, this work may expand our understanding of how KSHV evades host inflammation and immunity for its survival in vivo.
Importance: MyD88 is an important molecule for IL-1-mediated inflammation and Toll-like receptor (TLR) signaling. This work shows that KSHV inhibits MyD88 expression through a novel mechanism. KSHV RTA may bind to MyD88 RNA, suppresses RNA synthesis of MyD88, and inhibits IL-1-mediated signaling. This work may expand our understanding of how KSHV evades host inflammation and immunity.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





Similar articles
-
Kaposi's sarcoma-associated herpesvirus-encoded replication and transcription activator impairs innate immunity via ubiquitin-mediated degradation of myeloid differentiation factor 88.J Virol. 2015 Jan;89(1):415-27. doi: 10.1128/JVI.02591-14. Epub 2014 Oct 15. J Virol. 2015. PMID: 25320320 Free PMC article.
-
Kaposi sarcoma-associated herpesvirus degrades cellular Toll-interleukin-1 receptor domain-containing adaptor-inducing beta-interferon (TRIF).J Biol Chem. 2011 Mar 11;286(10):7865-7872. doi: 10.1074/jbc.M110.191452. Epub 2011 Jan 6. J Biol Chem. 2011. PMID: 21212282 Free PMC article.
-
Guanylate-Binding Protein 1 Inhibits Nuclear Delivery of Kaposi's Sarcoma-Associated Herpesvirus Virions by Disrupting Formation of Actin Filament.J Virol. 2017 Jul 27;91(16):e00632-17. doi: 10.1128/JVI.00632-17. Print 2017 Aug 15. J Virol. 2017. PMID: 28592529 Free PMC article.
-
The role of Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8 regulator of transcription activation (RTA) in control of gene expression.Oncogene. 2003 Aug 11;22(33):5150-63. doi: 10.1038/sj.onc.1206555. Oncogene. 2003. PMID: 12910252 Review.
-
KSHV reactivation and novel implications of protein isomerization on lytic switch control.Viruses. 2015 Jan 12;7(1):72-109. doi: 10.3390/v7010072. Viruses. 2015. PMID: 25588053 Free PMC article. Review.
Cited by
-
MyD88 Regulates LPS-induced NF-ĸB/MAPK Cytokines and Promotes Inflammation and Malignancy in Colorectal Cancer Cells.Cancer Genomics Proteomics. 2019 Nov-Dec;16(6):409-419. doi: 10.21873/cgp.20145. Cancer Genomics Proteomics. 2019. PMID: 31659096 Free PMC article.
-
KSHV: Immune Modulation and Immunotherapy.Front Immunol. 2020 Feb 7;10:3084. doi: 10.3389/fimmu.2019.03084. eCollection 2019. Front Immunol. 2020. PMID: 32117196 Free PMC article. Review.
-
Novel immune checkpoint-related gene model to predict prognosis and treatment responsiveness in low-grade gliomas.Heliyon. 2023 Sep 14;9(9):e20178. doi: 10.1016/j.heliyon.2023.e20178. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809899 Free PMC article.
-
MyD88 mediates colorectal cancer cell proliferation, migration and invasion via NF‑κB/AP‑1 signaling pathway.Int J Mol Med. 2020 Jan;45(1):131-140. doi: 10.3892/ijmm.2019.4390. Epub 2019 Oct 31. Int J Mol Med. 2020. PMID: 31746347 Free PMC article.
-
Antiviral activity of 20(R)-ginsenoside Rh2 against murine gammaherpesvirus.J Ginseng Res. 2017 Oct;41(4):496-502. doi: 10.1016/j.jgr.2016.08.010. Epub 2016 Sep 1. J Ginseng Res. 2017. PMID: 29021696 Free PMC article.
References
-
- Chang Y, Moore PS. 1996. Kaposi's sarcoma (KS)-associated herpesvirus and its role in KS. Infect Agents Dis 5:215–222. - PubMed
-
- Moore P, Chang Y. 2001. Kaposi's sarcoma-associated herpesvirus, p 2803–2833. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 4th ed Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

