Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera
- PMID: 26468324
- PMCID: PMC4672383
- DOI: 10.1126/scitranslmed.aac4241
Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera
Abstract
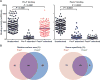
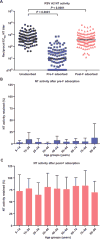
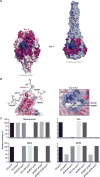
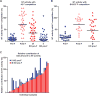
Respiratory syncytial virus (RSV) is estimated to claim more lives among infants <1 year old than any other single pathogen, except malaria, and poses a substantial global health burden. Viral entry is mediated by a type I fusion glycoprotein (F) that transitions from a metastable prefusion (pre-F) to a stable postfusion (post-F) trimer. A highly neutralization-sensitive epitope, antigenic site Ø, is found only on pre-F. We determined what fraction of neutralizing (NT) activity in human sera is dependent on antibodies specific for antigenic site Ø or other antigenic sites on F in healthy subjects from ages 7 to 93 years. Adsorption of individual sera with stabilized pre-F protein removed >90% of NT activity and depleted binding antibodies to both F conformations. In contrast, adsorption with post-F removed ~30% of NT activity, and binding antibodies to pre-F were retained. These findings were consistent across all age groups. Protein competition neutralization assays with pre-F mutants in which sites Ø or II were altered to knock out binding of antibodies to the corresponding sites showed that these sites accounted for ~35 and <10% of NT activity, respectively. Binding competition assays with monoclonal antibodies (mAbs) indicated that the amount of site Ø-specific antibodies correlated with NT activity, whereas the magnitude of binding competed by site II mAbs did not correlate with neutralization. Our results indicate that RSV NT activity in human sera is primarily derived from pre-F-specific antibodies, and therefore, inducing or boosting NT activity by vaccination will be facilitated by using pre-F antigens that preserve site Ø.
Copyright © 2015, American Association for the Advancement of Science.
Conflict of interest statement
Figures

Similar articles
-
Characterization of Epitope-Specific Anti-Respiratory Syncytial Virus (Anti-RSV) Antibody Responses after Natural Infection and after Vaccination with Formalin-Inactivated RSV.J Virol. 2016 Jun 10;90(13):5965-5977. doi: 10.1128/JVI.00235-16. Print 2016 Jul 1. J Virol. 2016. PMID: 27099320 Free PMC article.
-
Discovery of a Prefusion Respiratory Syncytial Virus F-Specific Monoclonal Antibody That Provides Greater In Vivo Protection than the Murine Precursor of Palivizumab.J Virol. 2017 Jul 12;91(15):e00176-17. doi: 10.1128/JVI.00176-17. Print 2017 Aug 1. J Virol. 2017. PMID: 28539438 Free PMC article.
-
A novel pre-fusion conformation-specific neutralizing epitope on the respiratory syncytial virus fusion protein.Nat Microbiol. 2017 Jan 30;2:16271. doi: 10.1038/nmicrobiol.2016.271. Nat Microbiol. 2017. PMID: 28134924 Free PMC article.
-
Neutralizing epitopes on the respiratory syncytial virus fusion glycoprotein.Curr Opin Virol. 2015 Apr;11:70-5. doi: 10.1016/j.coviro.2015.03.002. Epub 2015 Mar 26. Curr Opin Virol. 2015. PMID: 25819327 Free PMC article. Review.
-
Clinical Potential of Prefusion RSV F-specific Antibodies.Trends Microbiol. 2018 Mar;26(3):209-219. doi: 10.1016/j.tim.2017.09.009. Epub 2017 Oct 17. Trends Microbiol. 2018. PMID: 29054341 Review.
Cited by
-
Surface-modified measles vaccines encoding oligomeric, prefusion-stabilized SARS-CoV-2 spike glycoproteins boost neutralizing antibody responses to Omicron and historical variants, independent of measles seropositivity.mBio. 2024 Feb 14;15(2):e0292823. doi: 10.1128/mbio.02928-23. Epub 2024 Jan 9. mBio. 2024. PMID: 38193729 Free PMC article.
-
Safety and Immunogenicity of an mRNA-Based RSV Vaccine Including a 12-Month Booster in a Phase 1 Clinical Trial in Healthy Older Adults.J Infect Dis. 2024 Sep 23;230(3):e647-e656. doi: 10.1093/infdis/jiae081. J Infect Dis. 2024. PMID: 38385566 Free PMC article. Clinical Trial.
-
Antibody levels against respiratory syncytial virus fusion protein conformations and lack of association with life-threatening infection in previously healthy infants.Vaccine. 2024 Nov 14;42(25):126119. doi: 10.1016/j.vaccine.2024.07.020. Epub 2024 Jul 12. Vaccine. 2024. PMID: 39003106
-
Protective antibodies against human parainfluenza virus type 3 infection.MAbs. 2021 Jan-Dec;13(1):1912884. doi: 10.1080/19420862.2021.1912884. MAbs. 2021. PMID: 33876699 Free PMC article.
-
Vaccination with prefusion-stabilized respiratory syncytial virus fusion protein induces genetically and antigenically diverse antibody responses.Immunity. 2021 Apr 13;54(4):769-780.e6. doi: 10.1016/j.immuni.2021.03.004. Epub 2021 Apr 5. Immunity. 2021. PMID: 33823129 Free PMC article. Clinical Trial.
References
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. - PubMed
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simões EA, Rudan I, Weber MW, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet. 2010;375:1545–1555. - PMC - PubMed
-
- Anderson LJ. Respiratory syncytial virus vaccine development. Semin Immunol. 2013;25:160–171. - PubMed
-
- Caldera LJ, González-Reyes L, García-Barreno B, Wharton SA, Skehel JJ, Wiley DC, Melerob JA. Electron microscopy of the human respiratory syncytial virus fusion protein and complexes that it forms with monoclonal antibodies. Virology. 2000;271:122–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

