EBV Persistence--Introducing the Virus
- PMID: 26424647
- PMCID: PMC5125397
- DOI: 10.1007/978-3-319-22822-8_8
EBV Persistence--Introducing the Virus
Abstract
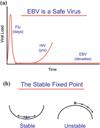
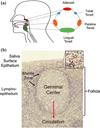
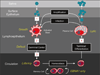
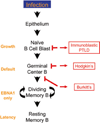
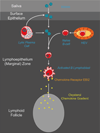
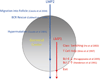
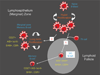
Persistent infection by EBV is explained by the germinal center model (GCM) which provides a satisfying and currently the only explanation for EBVs disparate biology. Since the GCM touches on every aspect of the virus, this chapter will serve as an introduction to the subsequent chapters. EBV is B lymphotropic, and its biology closely follows that of normal mature B lymphocytes. The virus persists quiescently in resting memory B cells for the lifetime of the host in a non-pathogenic state that is also invisible to the immune response. To access this compartment, the virus infects naïve B cells in the lymphoepithelium of the tonsils and activates these cells using the growth transcription program. These cells migrate to the GC where they switch to a more limited transcription program, the default program, which helps rescue them into the memory compartment where the virus persists. For egress, the infected memory cells return to the lymphoepithelium where they occasionally differentiate into plasma cells activating viral replication. The released virus can either infect more naïve B cells or be amplified in the epithelium for shedding. This cycle of infection and the quiescent state in memory B cells allow for lifetime persistence at a very low level that is remarkably stable over time. Mathematically, this is a stable fixed point where the mechanisms regulating persistence drive the state back to equilibrium when perturbed. This is the GCM of EBV persistence. Other possible sites and mechanisms of persistence will also be discussed.
Figures


Similar articles
-
The pathogenesis of Epstein-Barr virus persistent infection.Curr Opin Virol. 2013 Jun;3(3):227-32. doi: 10.1016/j.coviro.2013.04.005. Epub 2013 May 15. Curr Opin Virol. 2013. PMID: 23683686 Free PMC article. Review.
-
The cycle of EBV infection explains persistence, the sizes of the infected cell populations and which come under CTL regulation.PLoS Pathog. 2013;9(10):e1003685. doi: 10.1371/journal.ppat.1003685. Epub 2013 Oct 17. PLoS Pathog. 2013. PMID: 24146621 Free PMC article.
-
The BHLF1 Locus of Epstein-Barr Virus Contributes to Viral Latency and B-Cell Immortalization.J Virol. 2020 Aug 17;94(17):e01215-20. doi: 10.1128/JVI.01215-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32581094 Free PMC article.
-
The ins and outs of EBV infection.Trends Microbiol. 2000 Apr;8(4):185-9. doi: 10.1016/s0966-842x(00)01742-x. Trends Microbiol. 2000. PMID: 10754578 Review.
-
Epstein-Barr virus latency: LMP2, a regulator or means for Epstein-Barr virus persistence?Adv Cancer Res. 2000;79:175-200. doi: 10.1016/s0065-230x(00)79006-3. Adv Cancer Res. 2000. PMID: 10818681 Review.
Cited by
-
Analyzing the Impact of the Highest Expressed Epstein-Barr Virus-Encoded microRNAs on the Host Cell Transcriptome.Int J Mol Sci. 2024 Jul 17;25(14):7838. doi: 10.3390/ijms25147838. Int J Mol Sci. 2024. PMID: 39063079 Free PMC article.
-
Epstein-Barr Virus BARF1 Is Expressed in Lung Cancer and Is Associated with Cancer Progression.Cells. 2024 Sep 19;13(18):1578. doi: 10.3390/cells13181578. Cells. 2024. PMID: 39329759 Free PMC article.
-
Roles of Lytic Viral Replication and Co-Infections in the Oncogenesis and Immune Control of the Epstein-Barr Virus.Cancers (Basel). 2021 May 10;13(9):2275. doi: 10.3390/cancers13092275. Cancers (Basel). 2021. PMID: 34068598 Free PMC article. Review.
-
Concomitant Cytotoxic Effector Differentiation of CD4+ and CD8+ T Cells in Response to EBV-Infected B Cells.Cancers (Basel). 2022 Aug 25;14(17):4118. doi: 10.3390/cancers14174118. Cancers (Basel). 2022. PMID: 36077655 Free PMC article.
-
Epstein-Barr Virus-Associated Malignancies: Roles of Viral Oncoproteins in Carcinogenesis.Front Oncol. 2018 Aug 2;8:265. doi: 10.3389/fonc.2018.00265. eCollection 2018. Front Oncol. 2018. PMID: 30116721 Free PMC article. Review.
References
-
- Allday MJ, Crawford DH, Griffin BE. Epstein-Barr virus latent gene expression during the initiation of B cell immortalization. J Gen Virol. 1989;70:1755–1764. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

