Kaposi's Sarcoma-Associated Herpesvirus Viral Interferon Regulatory Factor 1 Interacts with a Member of the Interferon-Stimulated Gene 15 Pathway
- PMID: 26355087
- PMCID: PMC4645652
- DOI: 10.1128/JVI.01482-15
Kaposi's Sarcoma-Associated Herpesvirus Viral Interferon Regulatory Factor 1 Interacts with a Member of the Interferon-Stimulated Gene 15 Pathway
Abstract
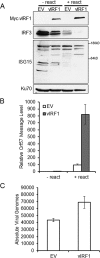
Kaposi's sarcoma-associated herpesvirus (KSHV) is a gammaherpesvirus known to establish lifelong latency in the human host. We and others have previously shown that three KSHV homologs of cellular interferon regulatory factors (IRFs), known as viral IRFs (vIRFs), participate in evasion of the host interferon (IFN) response. We report that vIRF1 interacts with the cellular interferon-stimulated gene 15 (ISG15) E3 ligase, HERC5, in the context of Toll-like receptor 3 (TLR3) activation and IFN induction. The ISG15 protein is covalently conjugated to target proteins upon activation of the interferon response. Interaction between vIRF1 and HERC5 was confirmed by immunoprecipitation, and the region between amino acids 224 and 349 of vIRF1 was required for interaction with HERC5. We further report that expression of vIRF1 in the context of TLR3 activation results in decreased ISG15 conjugation of proteins. Specifically, TLR3-induced ISG15 conjugation and protein levels of cellular IRF3, a known ISG15 target, were decreased in the presence of vIRF1 compared to the control. vIRF1 itself was also identified as a target of ISG15 conjugation. KSHV-infected cells exhibited increased ISG15 conjugation upon reactivation from latency in coordination with increased IFN. Furthermore, knockdown of ISG15 in latently infected cells resulted in a higher level of KSHV reactivation and an increase in infectious virus. These data suggest that the KSHV vIRF1 protein affects ISG15 conjugation and interferon responses and may contribute to effective KSHV replication.
Importance: The KSHV vIRF1 protein can inhibit interferon activation in response to viral infection. We identified a cellular protein named HERC5, which is the major ligase for ISG15, as a vIRF1 binding partner. vIRF1 association with HERC5 altered ISG15 modification of cellular proteins, and knockdown of ISG15 augmented reactivation of KSHV from latency.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


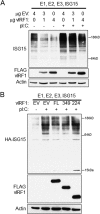
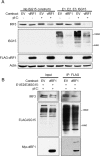
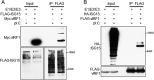
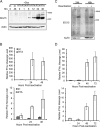
Similar articles
-
The viral interferon regulatory factors of kaposi's sarcoma-associated herpesvirus differ in their inhibition of interferon activation mediated by toll-like receptor 3.J Virol. 2013 Jan;87(2):798-806. doi: 10.1128/JVI.01851-12. Epub 2012 Oct 31. J Virol. 2013. PMID: 23115281 Free PMC article.
-
Genome-Wide Mapping of the Binding Sites and Structural Analysis of Kaposi's Sarcoma-Associated Herpesvirus Viral Interferon Regulatory Factor 2 Reveal that It Is a DNA-Binding Transcription Factor.J Virol. 2015 Nov 4;90(3):1158-68. doi: 10.1128/JVI.01392-15. Print 2016 Feb 1. J Virol. 2015. PMID: 26537687 Free PMC article.
-
Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses.Proc Natl Acad Sci U S A. 2015 Aug 4;112(31):E4306-15. doi: 10.1073/pnas.1503831112. Epub 2015 Jul 21. Proc Natl Acad Sci U S A. 2015. PMID: 26199418 Free PMC article.
-
Coronaviral PLpro proteases and the immunomodulatory roles of conjugated versus free Interferon Stimulated Gene product-15 (ISG15).Semin Cell Dev Biol. 2022 Dec;132:16-26. doi: 10.1016/j.semcdb.2022.06.005. Epub 2022 Jun 25. Semin Cell Dev Biol. 2022. PMID: 35764457 Free PMC article. Review.
-
Distinct roles of Kaposi's sarcoma-associated herpesvirus-encoded viral interferon regulatory factors in inflammatory response and cancer.J Virol. 2013 Sep;87(17):9398-410. doi: 10.1128/JVI.03315-12. Epub 2013 Jun 19. J Virol. 2013. PMID: 23785197 Free PMC article. Review.
Cited by
-
Viral Ubiquitin and Ubiquitin-Like Deconjugases-Swiss Army Knives for Infection.Biomolecules. 2020 Aug 1;10(8):1137. doi: 10.3390/biom10081137. Biomolecules. 2020. PMID: 32752270 Free PMC article. Review.
-
ISG15: its roles in SARS-CoV-2 and other viral infections.Trends Microbiol. 2023 Dec;31(12):1262-1275. doi: 10.1016/j.tim.2023.07.006. Epub 2023 Aug 10. Trends Microbiol. 2023. PMID: 37573184 Free PMC article. Review.
-
Transmembrane Protein pUL50 of Human Cytomegalovirus Inhibits ISGylation by Downregulating UBE1L.J Virol. 2018 Jul 17;92(15):e00462-18. doi: 10.1128/JVI.00462-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29743376 Free PMC article.
-
Innate Immune Responses to Herpesvirus Infection.Cells. 2021 Aug 18;10(8):2122. doi: 10.3390/cells10082122. Cells. 2021. PMID: 34440891 Free PMC article. Review.
-
Pathogenesis of Human Gammaherpesviruses: Recent Advances.Curr Clin Microbiol Rep. 2019;6(3):166-174. doi: 10.1007/s40588-019-00127-2. Epub 2019 Aug 1. Curr Clin Microbiol Rep. 2019. PMID: 33134035 Free PMC article.
References
-
- Loeb KR, Haas AL. 1992. The interferon-inducible 15-kDa ubiquitin homolog conjugates to intracellular proteins. J Biol Chem 267:7806–7813. - PubMed
-
- Hemelaar J, Borodovsky A, Kessler BM, Reverter D, Cook J, Kolli N, Gan-Erdene T, Wilkinson KD, Gill G, Lima CD, Ploegh HL, Ovaa H. 2004. Specific and covalent targeting of conjugating and deconjugating enzymes of ubiquitin-like proteins. Mol Cell Biol 24:84–95. doi:10.1128/MCB.24.1.84-95.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

