Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia
- PMID: 26333935
- PMCID: PMC5909068
- DOI: 10.1126/scitranslmed.aac5415
Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia
Abstract
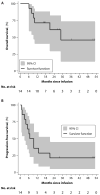
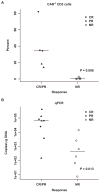
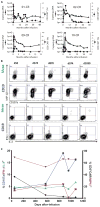
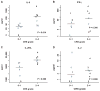
Patients with multiply relapsed or refractory chronic lymphocytic leukemia (CLL) have a poor prognosis. Chimeric antigen receptor (CAR)-modified T cells targeting CD19 have the potential to improve on the low complete response rates with conventional therapies by inducing sustained remissions in patients with refractory B cell malignancies. We previously reported preliminary results on three patients with refractory CLL. We report the mature results from our initial trial using CAR-modified T cells to treat 14 patients with relapsed and refractory CLL. Autologous T cells transduced with a CD19-directed CAR (CTL019) lentiviral vector were infused into patients with relapsed/refractory CLL at doses of 0.14 × 10(8) to 11 × 10(8) CTL019 cells (median, 1.6 × 10(8) cells). Patients were monitored for toxicity, response, expansion, and persistence of circulating CTL019 T cells. The overall response rate in these heavily pretreated CLL patients was 8 of 14 (57%), with 4 complete remissions (CR) and 4 partial remissions (PR). The in vivo expansion of the CAR T cells correlated with clinical responses, and the CAR T cells persisted and remained functional beyond 4 years in the first two patients achieving CR. No patient in CR has relapsed. All responding patients developed B cell aplasia and experienced cytokine release syndrome, coincident with T cell proliferation. Minimal residual disease was not detectable in patients who achieved CR, suggesting that disease eradication may be possible in some patients with advanced CLL.
Copyright © 2015, American Association for the Advancement of Science.
Conflict of interest statement
Figures

Similar articles
-
Chimeric antigen receptor T cells for sustained remissions in leukemia.N Engl J Med. 2014 Oct 16;371(16):1507-17. doi: 10.1056/NEJMoa1407222. N Engl J Med. 2014. PMID: 25317870 Free PMC article. Clinical Trial.
-
Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor-Modified T Cells After Failure of Ibrutinib.J Clin Oncol. 2017 Sep 10;35(26):3010-3020. doi: 10.1200/JCO.2017.72.8519. Epub 2017 Jul 17. J Clin Oncol. 2017. PMID: 28715249 Free PMC article. Clinical Trial.
-
Safety and tolerability of conditioning chemotherapy followed by CD19-targeted CAR T cells for relapsed/refractory CLL.JCI Insight. 2019 Apr 2;5(9):e122627. doi: 10.1172/jci.insight.122627. JCI Insight. 2019. PMID: 30938714 Free PMC article. Clinical Trial.
-
CAR T Cell Therapy in Acute Lymphoblastic Leukemia and Potential for Chronic Lymphocytic Leukemia.Curr Treat Options Oncol. 2016 Jun;17(6):28. doi: 10.1007/s11864-016-0406-4. Curr Treat Options Oncol. 2016. PMID: 27098534 Review.
-
CAR T-cells merge into the fast lane of cancer care.Am J Hematol. 2016 Jan;91(1):146-50. doi: 10.1002/ajh.24238. Am J Hematol. 2016. PMID: 26574400 Review.
Cited by
-
The CD28-Transmembrane Domain Mediates Chimeric Antigen Receptor Heterodimerization With CD28.Front Immunol. 2021 Mar 23;12:639818. doi: 10.3389/fimmu.2021.639818. eCollection 2021. Front Immunol. 2021. PMID: 33833759 Free PMC article.
-
Dawn of Chimeric Antigen Receptor T Cell Therapy in Non-Hodgkin Lymphoma.Adv Cell Gene Ther. 2018 Nov;1(3):e23. doi: 10.1002/acg2.23. Epub 2018 Oct 7. Adv Cell Gene Ther. 2018. PMID: 33043278 Free PMC article.
-
Current Advances in CAR T Cell Therapy for Malignant Mesothelioma.J Cell Immunol. 2020;2(4):192-200. doi: 10.33696/immunology.2.042. J Cell Immunol. 2020. PMID: 32914147 Free PMC article.
-
Planes, Trains, and Automobiles: Perspectives on CAR T Cells and Other Cellular Therapies for Hematologic Malignancies.Curr Hematol Malig Rep. 2016 Aug;11(4):318-25. doi: 10.1007/s11899-016-0330-5. Curr Hematol Malig Rep. 2016. PMID: 27136938 Free PMC article. Review.
-
Selection, Expansion, and Unique Pretreatment of Allogeneic Human Natural Killer Cells with Anti-CD38 Monoclonal Antibody for Efficient Multiple Myeloma Treatment.Cells. 2021 Apr 21;10(5):967. doi: 10.3390/cells10050967. Cells. 2021. PMID: 33919155 Free PMC article.
References
-
- Dreger P, Schetelig J, Andersen N, Corradini P, van Gelder M, Gribben J, Kimby E, Michallet M, Moreno C, Stilgenbauer S, Montserrat E. European Research Initiative on CLL (ERIC) and the European Society for Blood and Marrow Transplantation (EBMT), Managing high-risk CLL during transition to a new treatment era: Stem cell transplantation or novel agents? Blood. 2014;124:3841–3849. - PMC - PubMed
-
- Brown JR. The treatment of relapsed refractory chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:110–118. - PubMed
-
- Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. - PMC - PubMed
-
- Park JR, DiGiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, Meechoovet HB, Bautista C, Chang WC, Ostberg JR, Jensen MC. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. - PubMed
-
- Lamers CHJ, Sleijfer S, Vulto AG, Kruit WHJ, Kliffen M, Debets R, Gratama JW, Stoter G, Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: First clinical experience. J Clin Oncol. 2006;24:e20–e22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

