Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer's disease pathology
- PMID: 26284939
- PMCID: PMC4557123
- DOI: 10.1038/ncomms8967
Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer's disease pathology
Abstract
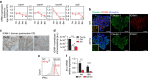
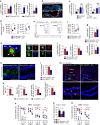
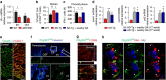
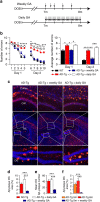
Alzheimer's disease (AD) is a neurodegenerative disorder in which chronic neuroinflammation contributes to disease escalation. Nevertheless, while immunosuppressive drugs have repeatedly failed in treating this disease, recruitment of myeloid cells to the CNS was shown to play a reparative role in animal models. Here we show, using the 5XFAD AD mouse model, that transient depletion of Foxp3(+) regulatory T cells (Tregs), or pharmacological inhibition of their activity, is followed by amyloid-β plaque clearance, mitigation of the neuroinflammatory response and reversal of cognitive decline. We further show that transient Treg depletion affects the brain's choroid plexus, a selective gateway for immune cell trafficking to the CNS, and is associated with subsequent recruitment of immunoregulatory cells, including monocyte-derived macrophages and Tregs, to cerebral sites of plaque pathology. Our findings suggest targeting Treg-mediated systemic immunosuppression for treating AD.
Figures

Similar articles
-
Regulatory T cells decrease C3-positive reactive astrocytes in Alzheimer-like pathology.J Neuroinflammation. 2023 Mar 8;20(1):64. doi: 10.1186/s12974-023-02702-3. J Neuroinflammation. 2023. PMID: 36890536 Free PMC article.
-
Therapeutic effects of glatiramer acetate and grafted CD115⁺ monocytes in a mouse model of Alzheimer's disease.Brain. 2015 Aug;138(Pt 8):2399-422. doi: 10.1093/brain/awv150. Epub 2015 Jun 6. Brain. 2015. PMID: 26049087 Free PMC article.
-
Neuroprotective effects of CD4+CD25+Foxp3+ regulatory T cells in a 3xTg-AD Alzheimer's disease model.Oncotarget. 2016 Oct 25;7(43):69347-69357. doi: 10.18632/oncotarget.12469. Oncotarget. 2016. PMID: 27713140 Free PMC article.
-
The regulation of immune tolerance by FOXP3.Nat Rev Immunol. 2017 Nov;17(11):703-717. doi: 10.1038/nri.2017.75. Epub 2017 Jul 31. Nat Rev Immunol. 2017. PMID: 28757603 Free PMC article. Review.
-
Regulatory T Cells: the Many Faces of Foxp3.J Clin Immunol. 2019 Oct;39(7):623-640. doi: 10.1007/s10875-019-00684-7. Epub 2019 Sep 2. J Clin Immunol. 2019. PMID: 31478130 Free PMC article. Review.
Cited by
-
α-Synuclein vaccination modulates regulatory T cell activation and microglia in the absence of brain pathology.J Neuroinflammation. 2016 Apr 7;13(1):74. doi: 10.1186/s12974-016-0532-8. J Neuroinflammation. 2016. PMID: 27055651 Free PMC article.
-
CD8+ T cells exacerbate AD-like symptoms in mouse model of amyloidosis.Brain Behav Immun. 2024 Nov;122:444-455. doi: 10.1016/j.bbi.2024.08.045. Epub 2024 Aug 25. Brain Behav Immun. 2024. PMID: 39191349
-
Dwellers and Trespassers: Mononuclear Phagocytes at the Borders of the Central Nervous System.Front Immunol. 2021 Mar 5;11:609921. doi: 10.3389/fimmu.2020.609921. eCollection 2020. Front Immunol. 2021. PMID: 33746939 Free PMC article. Review.
-
C9orf72 deficiency promotes microglial-mediated synaptic loss in aging and amyloid accumulation.Neuron. 2021 Jul 21;109(14):2275-2291.e8. doi: 10.1016/j.neuron.2021.05.020. Epub 2021 Jun 15. Neuron. 2021. PMID: 34133945 Free PMC article.
-
Aging-associated deficit in CCR7 is linked to worsened glymphatic function, cognition, neuroinflammation, and β-amyloid pathology.Sci Adv. 2021 May 21;7(21):eabe4601. doi: 10.1126/sciadv.abe4601. Print 2021 May. Sci Adv. 2021. PMID: 34020948 Free PMC article.
References
-
- Hardy J. & Selkoe D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002). - PubMed
-
- Weiner H. L. & Frenkel D. Immunology and immunotherapy of Alzheimer's disease. Nat. Rev. Immunol. 6, 404–416 (2006). - PubMed
-
- Anti-inflammatory drugs fall short in Alzheimer's disease. Nat. Med. 14, 916 (2008). - PubMed
-
- Group A. R. et al.. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology 68, 1800–1808 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

