An In-Depth Comparison of Latency-Reversing Agent Combinations in Various In Vitro and Ex Vivo HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression
- PMID: 26225566
- PMCID: PMC4520688
- DOI: 10.1371/journal.ppat.1005063
An In-Depth Comparison of Latency-Reversing Agent Combinations in Various In Vitro and Ex Vivo HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression
Abstract
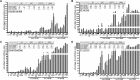
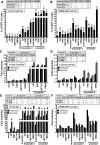
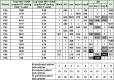
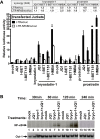
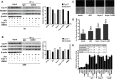
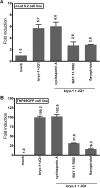
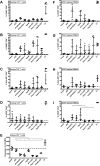
The persistence of latently infected cells in patients under combinatory antiretroviral therapy (cART) is a major hurdle to HIV-1 eradication. Strategies to purge these reservoirs are needed and activation of viral gene expression in latently infected cells is one promising strategy. Bromodomain and Extraterminal (BET) bromodomain inhibitors (BETi) are compounds able to reactivate latent proviruses in a positive transcription elongation factor b (P-TEFb)-dependent manner. In this study, we tested the reactivation potential of protein kinase C (PKC) agonists (prostratin, bryostatin-1 and ingenol-B), which are known to activate NF-κB signaling pathway as well as P-TEFb, used alone or in combination with P-TEFb-releasing agents (HMBA and BETi (JQ1, I-BET, I-BET151)). Using in vitro HIV-1 post-integration latency model cell lines of T-lymphoid and myeloid lineages, we demonstrated that PKC agonists and P-TEFb-releasing agents alone acted as potent latency-reversing agents (LRAs) and that their combinations led to synergistic activation of HIV-1 expression at the viral mRNA and protein levels. Mechanistically, combined treatments led to higher activations of P-TEFb and NF-κB than the corresponding individual drug treatments. Importantly, we observed in ex vivo cultures of CD8+-depleted PBMCs from 35 cART-treated HIV-1+ aviremic patients that the percentage of reactivated cultures following combinatory bryostatin-1+JQ1 treatment was identical to the percentage observed with anti-CD3+anti-CD28 antibodies positive control stimulation. Remarkably, in ex vivo cultures of resting CD4+ T cells isolated from 15 HIV-1+ cART-treated aviremic patients, the combinations bryostatin-1+JQ1 and ingenol-B+JQ1 released infectious viruses to levels similar to that obtained with the positive control stimulation. The potent effects of these two combination treatments were already detected 24 hours post-stimulation. These results constitute the first demonstration of LRA combinations exhibiting such a potent effect and represent a proof-of-concept for the co-administration of two different types of LRAs as a potential strategy to reduce the size of the latent HIV-1 reservoirs.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: LFP is a share holder of Kyolab laboratories. He also has a contact with Amazonia Fitomedicamentos to develop the ingenol derivatives. This does not alter the authors' adherence to Plos Pathogens policies on sharing data and materials.
Figures



Similar articles
-
Alternate NF-κB-Independent Signaling Reactivation of Latent HIV-1 Provirus.J Virol. 2019 Aug 28;93(18):e00495-19. doi: 10.1128/JVI.00495-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31243131 Free PMC article.
-
A New Quinoline BRD4 Inhibitor Targets a Distinct Latent HIV-1 Reservoir for Reactivation from Other "Shock" Drugs.J Virol. 2018 Apr 27;92(10):e02056-17. doi: 10.1128/JVI.02056-17. Print 2018 May 15. J Virol. 2018. PMID: 29343578 Free PMC article.
-
Specific Activation In Vivo of HIV-1 by a Bromodomain Inhibitor from Monocytic Cells in Humanized Mice under Antiretroviral Therapy.J Virol. 2019 May 29;93(12):e00233-19. doi: 10.1128/JVI.00233-19. Print 2019 Jun 15. J Virol. 2019. PMID: 30971469 Free PMC article.
-
Molecular mechanisms of HIV latency.J Clin Invest. 2016 Feb;126(2):448-54. doi: 10.1172/JCI80565. Epub 2016 Jan 5. J Clin Invest. 2016. PMID: 26731470 Free PMC article. Review.
-
The cell biology of HIV-1 latency and rebound.Retrovirology. 2024 Apr 5;21(1):6. doi: 10.1186/s12977-024-00639-w. Retrovirology. 2024. PMID: 38580979 Free PMC article. Review.
Cited by
-
Engineering CAR T Cells to Target the HIV Reservoir.Front Cell Infect Microbiol. 2020 Aug 13;10:410. doi: 10.3389/fcimb.2020.00410. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32903563 Free PMC article. Review.
-
Combinatorial Use of Both Epigenetic and Non-Epigenetic Mechanisms to Efficiently Reactivate HIV Latency.Int J Mol Sci. 2021 Apr 2;22(7):3697. doi: 10.3390/ijms22073697. Int J Mol Sci. 2021. PMID: 33918134 Free PMC article. Review.
-
Opposite transcriptional regulation of integrated vs unintegrated HIV genomes by the NF-κB pathway.Sci Rep. 2016 May 11;6:25678. doi: 10.1038/srep25678. Sci Rep. 2016. PMID: 27167871 Free PMC article.
-
The Effect of Latency Reversal Agents on Primary CD8+ T Cells: Implications for Shock and Kill Strategies for Human Immunodeficiency Virus Eradication.EBioMedicine. 2016 Jun;8:217-229. doi: 10.1016/j.ebiom.2016.04.019. Epub 2016 Apr 18. EBioMedicine. 2016. PMID: 27428432 Free PMC article.
-
Reiterative Enrichment and Authentication of CRISPRi Targets (REACT) identifies the proteasome as a key contributor to HIV-1 latency.PLoS Pathog. 2019 Jan 15;15(1):e1007498. doi: 10.1371/journal.ppat.1007498. eCollection 2019 Jan. PLoS Pathog. 2019. PMID: 30645648 Free PMC article.
References
-
- Smit C, Geskus R, Walker S, Sabin C, Coutinho R, et al. (2006) Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS 20: 741–749. - PubMed
-
- Friis-Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, et al. (2003) Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 349: 1993–2003. - PubMed
-
- Blankson JN, Persaud D, Siliciano RF (2002) The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med 53: 557–593. - PubMed
-
- Crowe S, Zhu T, Muller WA (2003) The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol 74: 635–641. - PubMed
-
- Lewin SR, Kirihara J, Sonza S, Irving L, Mills J, et al. (1998) HIV-1 DNA and mRNA concentrations are similar in peripheral blood monocytes and alveolar macrophages in HIV-1-infected individuals. AIDS 12: 719–727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

