Fibroblast growth factor receptor signaling in hereditary and neoplastic disease: biologic and clinical implications
- PMID: 26224133
- PMCID: PMC4573649
- DOI: 10.1007/s10555-015-9579-8
Fibroblast growth factor receptor signaling in hereditary and neoplastic disease: biologic and clinical implications
Abstract
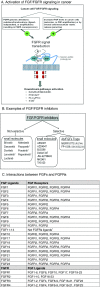
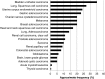
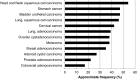
Fibroblast growth factors (FGFs) and their receptors (FGFRs) are transmembrane growth factor receptors with wide tissue distribution. FGF/FGFR signaling is involved in neoplastic behavior and also development, differentiation, growth, and survival. FGFR germline mutations (activating) can cause skeletal disorders, primarily dwarfism (generally mutations in FGFR3), and craniofacial malformation syndromes (usually mutations in FGFR1 and FGFR2); intriguingly, some of these activating FGFR mutations are also seen in human cancers. FGF/FGFR aberrations reported in cancers are mainly thought to be gain-of-function changes, and several cancers have high frequencies of FGFR alterations, including breast, bladder, or squamous cell carcinomas (lung and head and neck). FGF ligand aberrations (predominantly gene amplifications) are also frequently seen in cancers, in contrast to hereditary syndromes. There are several pharmacologic agents that have been or are being developed for inhibition of FGFR/FGF signaling. These include both highly selective inhibitors as well as multi-kinase inhibitors. Of note, only four agents (ponatinib, pazopanib, regorafenib, and recently lenvatinib) are FDA-approved for use in cancer, although the approval was not based on their activity against FGFR. Perturbations in the FGFR/FGF signaling are present in both inherited and malignant diseases. The development of potent inhibitors targeting FGF/FGFR may provide new tools against disorders caused by FGF/FGFR alterations.
Keywords: Cancer; Cancer therapy; FGF; FGFR; Genetics.
Figures
Similar articles
-
Fibroblast growth factor family aberrations in cancers: clinical and molecular characteristics.Cell Cycle. 2015;14(13):2121-8. doi: 10.1080/15384101.2015.1041691. Epub 2015 May 7. Cell Cycle. 2015. PMID: 25950492 Free PMC article.
-
Small molecule inhibition of fibroblast growth factor receptors in cancer.Cytokine Growth Factor Rev. 2013 Oct;24(5):467-75. doi: 10.1016/j.cytogfr.2013.05.002. Epub 2013 Jul 2. Cytokine Growth Factor Rev. 2013. PMID: 23830577 Review.
-
Implications of Fibroblast Growth Factors (FGFs) in Cancer: From Prognostic to Therapeutic Applications.Curr Drug Targets. 2019;20(8):852-870. doi: 10.2174/1389450120666190112145409. Curr Drug Targets. 2019. PMID: 30648505 Review.
-
Fibroblast growth factor (FGF), FGF receptor (FGFR), and cyclin D1 (CCND1) DNA methylation in head and neck squamous cell carcinomas is associated with transcriptional activity, gene amplification, human papillomavirus (HPV) status, and sensitivity to tyrosine kinase inhibitors.Clin Epigenetics. 2021 Dec 21;13(1):228. doi: 10.1186/s13148-021-01212-4. Clin Epigenetics. 2021. PMID: 34933671 Free PMC article.
-
The role of fibroblast growth factor receptor (FGFR) protein-tyrosine kinase inhibitors in the treatment of cancers including those of the urinary bladder.Pharmacol Res. 2020 Jan;151:104567. doi: 10.1016/j.phrs.2019.104567. Epub 2019 Nov 23. Pharmacol Res. 2020. PMID: 31770593 Review.
Cited by
-
Targeting the Oncogenic FGF-FGFR Axis in Gastric Carcinogenesis.Cells. 2019 Jun 25;8(6):637. doi: 10.3390/cells8060637. Cells. 2019. PMID: 31242658 Free PMC article. Review.
-
Gastric Cancer: Molecular Mechanisms, Novel Targets, and Immunotherapies: From Bench to Clinical Therapeutics.Cancers (Basel). 2023 Oct 20;15(20):5075. doi: 10.3390/cancers15205075. Cancers (Basel). 2023. PMID: 37894443 Free PMC article. Review.
-
Landscape of activating cancer mutations in FGFR kinases and their differential responses to inhibitors in clinical use.Oncotarget. 2016 Apr 26;7(17):24252-68. doi: 10.18632/oncotarget.8132. Oncotarget. 2016. PMID: 26992226 Free PMC article.
-
Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application.Oncotarget. 2017 Feb 28;8(9):16052-16074. doi: 10.18632/oncotarget.14109. Oncotarget. 2017. PMID: 28030802 Free PMC article. Review.
-
Emerging Targets for Systemic Treatment of Gastric Cancer: HER2 and Beyond.J Gastric Cancer. 2024 Jan;24(1):29-56. doi: 10.5230/jgc.2024.24.e6. J Gastric Cancer. 2024. PMID: 38225765 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

