Defining Effective Combinations of Immune Checkpoint Blockade and Oncolytic Virotherapy
- PMID: 26187615
- PMCID: PMC4681662
- DOI: 10.1158/1078-0432.CCR-14-2009
Defining Effective Combinations of Immune Checkpoint Blockade and Oncolytic Virotherapy
Abstract
Purpose: Recent data from randomized clinical trials with oncolytic viral therapies and with cancer immunotherapies have finally recapitulated the promise these platforms demonstrated in preclinical models. Perhaps the greatest advance with oncolytic virotherapy has been the appreciation of the importance of activation of the immune response in therapeutic activity. Meanwhile, the understanding that blockade of immune checkpoints (with antibodies that block the binding of PD1 to PDL1 or CTLA4 to B7-2) is critical for an effective antitumor immune response has revitalized the field of immunotherapy. The combination of immune activation using an oncolytic virus and blockade of immune checkpoints is therefore a logical next step.
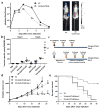
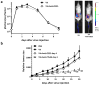
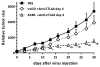
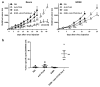
Experimental design: Here, we explore such combinations and demonstrate their potential to produce enhanced responses in mouse tumor models. Different combinations and regimens were explored in immunocompetent mouse models of renal and colorectal cancer. Bioluminescence imaging and immune assays were used to determine the mechanisms mediating synergistic or antagonistic combinations.
Results: Interaction between immune checkpoint inhibitors and oncolytic virotherapy was found to be complex, with correct selection of viral strain, antibody, and timing of the combination being critical for synergistic effects. Indeed, some combinations produced antagonistic effects and loss of therapeutic activity. A period of oncolytic viral replication and directed targeting of the immune response against the tumor were required for the most beneficial effects, with CD8(+) and NK, but not CD4(+) cells mediating the effects.
Conclusions: These considerations will be critical in the design of the inevitable clinical translation of these combination approaches. Clin Cancer Res; 21(24); 5543-51. ©2015 AACR.See related commentary by Slaney and Darcy, p. 5417.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures
Comment in
-
Releasing the Brake on Oncolytic Viral Therapy.Clin Cancer Res. 2015 Dec 15;21(24):5417-9. doi: 10.1158/1078-0432.CCR-15-1769. Epub 2015 Sep 16. Clin Cancer Res. 2015. PMID: 26378034
Similar articles
-
An Oncolytic Adenovirus Targeting Transforming Growth Factor β Inhibits Protumorigenic Signals and Produces Immune Activation: A Novel Approach to Enhance Anti-PD-1 and Anti-CTLA-4 Therapy.Hum Gene Ther. 2019 Sep;30(9):1117-1132. doi: 10.1089/hum.2019.059. Epub 2019 Jul 1. Hum Gene Ther. 2019. PMID: 31126191 Free PMC article.
-
Oncolytic VSV Primes Differential Responses to Immuno-oncology Therapy.Mol Ther. 2017 Aug 2;25(8):1917-1932. doi: 10.1016/j.ymthe.2017.05.006. Epub 2017 Jun 2. Mol Ther. 2017. PMID: 28578991 Free PMC article.
-
Engineering Newcastle Disease Virus as an Oncolytic Vector for Intratumoral Delivery of Immune Checkpoint Inhibitors and Immunocytokines.J Virol. 2020 Jan 17;94(3):e01677-19. doi: 10.1128/JVI.01677-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31694938 Free PMC article.
-
Immunotherapy for the treatment of breast cancer: checkpoint blockade, cancer vaccines, and future directions in combination immunotherapy.Clin Adv Hematol Oncol. 2016 Nov;14(11):922-933. Clin Adv Hematol Oncol. 2016. PMID: 27930644 Review.
-
Oncolytic viruses as engineering platforms for combination immunotherapy.Nat Rev Cancer. 2018 Jul;18(7):419-432. doi: 10.1038/s41568-018-0009-4. Nat Rev Cancer. 2018. PMID: 29695749 Review.
Cited by
-
Bovine pestivirus is a new alternative virus for multiple myeloma oncolytic virotherapy.J Hematol Oncol. 2020 Jul 11;13(1):89. doi: 10.1186/s13045-020-00919-w. J Hematol Oncol. 2020. PMID: 32653014 Free PMC article.
-
Tune Up In Situ Autovaccination against Solid Tumors with Oncolytic Viruses.Cancers (Basel). 2018 May 31;10(6):171. doi: 10.3390/cancers10060171. Cancers (Basel). 2018. PMID: 29857493 Free PMC article. Review.
-
Immunotherapy for Esophageal Squamous Cell Carcinoma.Curr Oncol Rep. 2017 May;19(5):33. doi: 10.1007/s11912-017-0590-9. Curr Oncol Rep. 2017. PMID: 28361224 Free PMC article. Review.
-
Advances and new frontiers for immunotherapy in colorectal cancer: Setting the stage for neoadjuvant success?Mol Ther Oncolytics. 2021 May 14;22:1-12. doi: 10.1016/j.omto.2021.05.001. eCollection 2021 Sep 24. Mol Ther Oncolytics. 2021. PMID: 34307839 Free PMC article. Review.
-
Modulation of the Intratumoral Immune Landscape by Oncolytic Herpes Simplex Virus Virotherapy.Front Oncol. 2017 Jun 26;7:136. doi: 10.3389/fonc.2017.00136. eCollection 2017. Front Oncol. 2017. PMID: 28695111 Free PMC article. Review.
References
-
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

