NF-κB Has a Direct Role in Inhibiting Bmp- and Wnt-Induced Matrix Protein Expression
- PMID: 26179215
- PMCID: PMC4713353
- DOI: 10.1002/jbmr.2592
NF-κB Has a Direct Role in Inhibiting Bmp- and Wnt-Induced Matrix Protein Expression
Abstract
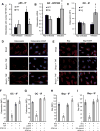
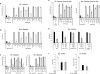
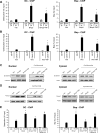
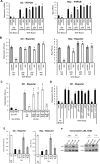
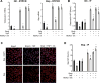
The host response to pathogens through nuclear factor κB (NF-κB) is an essential defense mechanism for eukaryotic organisms. NF-κB-mediated host responses inhibit bone and other connective tissue synthesis and are thought to affect the transcription of matrix proteins through multiple indirect pathways. We demonstrate that inhibiting NF-κB in osteoblasts increases osteocalcin expression in vivo in mice with periodontal disease. Mutating NF-κB binding sites on osteocalcin (OC) or bone sialoprotein (Bsp) promoters rescues the negative impact of NF-κB on their transcription and that NF-κB can inhibit Wnt- and Bmp-induced OC and Bsp transcription, even when protein synthesis is inhibited, indicating a direct effect of NF-κB. This inhibition depends on p65-p50 NF-κB heterodimer formation and deacetylation by HDAC1 but is not affected by the noncanonical NF-κB pathway. Moreover, NF-κB reduces Runx2 and β-catenin binding to OC/Bsp promoters independently of their nuclear localization. Thus, inflammatory signals stimulate the direct interaction of NF-κB with response elements to inhibit binding of β-catenin and Runx2 binding to nearby consensus sites and reduce expression of matrix proteins. This direct mechanism provides a new explanation for the rapid decrease in new bone formation after inflammation-related NF-κB activation.
Keywords: BMP; BONE FORMATION; INFLAMMATION; MATRIX PROTEINS; NF-κB; OSTEOBLASTS; TNFα; WNT.
© 2015 American Society for Bone and Mineral Research.
Conflict of interest statement
All authors state that they have no conflicts of interests.
Figures

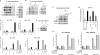
Similar articles
-
[NF-kappaB modulates activation of the BMP-2 gene by trichostatin A].Mol Biol (Mosk). 2008 Nov-Dec;42(6):990-6. Mol Biol (Mosk). 2008. PMID: 19140318 Russian.
-
WNT/β-catenin signaling inhibits CBP-mediated RelA acetylation and expression of proinflammatory NF-κB target genes.J Cell Sci. 2015 Jul 15;128(14):2430-6. doi: 10.1242/jcs.168542. Epub 2015 May 28. J Cell Sci. 2015. PMID: 26021349
-
Wnt/beta-catenin signaling regulates cytokine-induced human inducible nitric oxide synthase expression by inhibiting nuclear factor-kappaB activation in cancer cells.Cancer Res. 2009 May 1;69(9):3764-71. doi: 10.1158/0008-5472.CAN-09-0014. Epub 2009 Apr 21. Cancer Res. 2009. PMID: 19383900
-
Developmental pathways hijacked by osteosarcoma.Adv Exp Med Biol. 2014;804:93-118. doi: 10.1007/978-3-319-04843-7_5. Adv Exp Med Biol. 2014. PMID: 24924170 Review.
-
Insights on biology and pathology of HIF-1α/-2α, TGFβ/BMP, Wnt/β-catenin, and NF-κB pathways in osteoarthritis.Curr Pharm Des. 2012;18(22):3293-312. doi: 10.2174/1381612811209023293. Curr Pharm Des. 2012. PMID: 22646092 Review.
Cited by
-
Ablation of Wnt signaling in bone marrow stromal cells overcomes microenvironment-mediated drug resistance in acute myeloid leukemia.Sci Rep. 2024 Apr 10;14(1):8404. doi: 10.1038/s41598-024-58860-8. Sci Rep. 2024. PMID: 38600158 Free PMC article.
-
Topography-mediated immunomodulation in osseointegration; Ally or Enemy.Biomaterials. 2022 Dec;291:121903. doi: 10.1016/j.biomaterials.2022.121903. Epub 2022 Nov 9. Biomaterials. 2022. PMID: 36410109 Free PMC article. Review.
-
Empagliflozin ameliorates liver fibrosis in NASH rat model via targeting hepatic NF-κB/SOX9/OPN signaling and osteocalcin level.Naunyn Schmiedebergs Arch Pharmacol. 2024 May;397(5):3449-3459. doi: 10.1007/s00210-023-02826-6. Epub 2023 Nov 14. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37962587 Free PMC article.
-
Bench-to-bedside strategies for osteoporotic fracture: From osteoimmunology to mechanosensation.Bone Res. 2019 Aug 15;7:25. doi: 10.1038/s41413-019-0066-7. eCollection 2019. Bone Res. 2019. PMID: 31646015 Free PMC article. Review.
-
Transcriptional activity of vitamin D receptor in human periodontal ligament cells is diminished under inflammatory conditions.J Periodontol. 2021 Jan;92(1):137-148. doi: 10.1002/JPER.19-0541. Epub 2020 Jun 21. J Periodontol. 2021. PMID: 32474936 Free PMC article.
References
-
- Vitiello M, Galdiero M, Finamore E, Galdiero S, Galdiero M. NF-kappaB as a potential therapeutic target in microbial diseases. Mol Biosyst. 2012;8(4):1108–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

