Engineered antibody domains with significantly increased transcytosis and half-life in macaques mediated by FcRn
- PMID: 26179052
- PMCID: PMC4622838
- DOI: 10.1080/19420862.2015.1067353
Engineered antibody domains with significantly increased transcytosis and half-life in macaques mediated by FcRn
Abstract
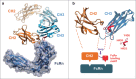
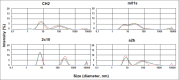
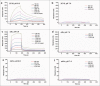
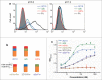
Engineered antibody domains (eAds) are promising candidate therapeutics but their half-life is relatively short partly due to weak or absent binding to the neonatal Fc receptor (FcRn). We developed a novel approach to increase the eAd binding to FcRn based on a combination of structure-based design, computational modeling and phage display methodologies. By using this approach, we identified 2 IgG1 CH2-derived eAds fused to a short FcRn-binding motif derived from IgG1 CH3 that exhibited greatly enhanced FcRn binding with strict pH dependency. Importantly, the increased affinity resulted in significantly enhanced FcRn-mediated epithelial transcytosis and prolonged elimination half-life (mean 44.1 hours) in cynomolgus macaques. These results demonstrate for the first time that the half-life of isolated eAds can be prolonged (optimized) by increasing their binding to FcRn while maintaining their small size, which has important implications for development of therapeutics, including eAd-drug conjugates with enhanced penetration in solid tissues.
Keywords: CH2; CH3; FcRn; antibody domains; half-life; macaques; transcytosis.
Figures

Similar articles
-
Engineered soluble monomeric IgG1 CH3 domain: generation, mechanisms of function, and implications for design of biological therapeutics.J Biol Chem. 2013 Aug 30;288(35):25154-25164. doi: 10.1074/jbc.M113.484154. Epub 2013 Jul 18. J Biol Chem. 2013. PMID: 23867459 Free PMC article.
-
Combined glyco- and protein-Fc engineering simultaneously enhance cytotoxicity and half-life of a therapeutic antibody.MAbs. 2014 Mar-Apr;6(2):422-36. doi: 10.4161/mabs.27854. Epub 2014 Jan 15. MAbs. 2014. PMID: 24492301 Free PMC article.
-
Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn).J Biol Chem. 2006 Aug 18;281(33):23514-24. doi: 10.1074/jbc.M604292200. Epub 2006 Jun 21. J Biol Chem. 2006. PMID: 16793771
-
Neonatal Fc receptor (FcRn): a novel target for therapeutic antibodies and antibody engineering.J Drug Target. 2014 May;22(4):269-78. doi: 10.3109/1061186X.2013.875030. Epub 2014 Jan 9. J Drug Target. 2014. PMID: 24404896 Review.
-
The multiple facets of FcRn in immunity.Immunol Rev. 2015 Nov;268(1):253-68. doi: 10.1111/imr.12331. Immunol Rev. 2015. PMID: 26497526 Review.
Cited by
-
Half-life extension of single-domain antibody-drug conjugates by albumin binding moiety enhances antitumor efficacy.MedComm (2020). 2024 May 9;5(5):e557. doi: 10.1002/mco2.557. eCollection 2024 May. MedComm (2020). 2024. PMID: 38737471 Free PMC article.
-
A neutralizing bispecific single-chain antibody against SARS-CoV-2 Omicron variant produced based on CR3022.Front Cell Infect Microbiol. 2023 May 3;13:1155293. doi: 10.3389/fcimb.2023.1155293. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37207187 Free PMC article.
-
In vivo pharmacokinetic enhancement of monomeric Fc and monovalent bispecific designs through structural guidance.Commun Biol. 2021 Sep 8;4(1):1048. doi: 10.1038/s42003-021-02565-5. Commun Biol. 2021. PMID: 34497355 Free PMC article.
-
The reduced form of the antibody CH2 domain.Protein Sci. 2021 Sep;30(9):1895-1903. doi: 10.1002/pro.4142. Epub 2021 Jun 16. Protein Sci. 2021. PMID: 34107549 Free PMC article.
-
In Translation: FcRn across the Therapeutic Spectrum.Int J Mol Sci. 2021 Mar 17;22(6):3048. doi: 10.3390/ijms22063048. Int J Mol Sci. 2021. PMID: 33802650 Free PMC article. Review.
References
-
- Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 2010; 9:767-74; PMID:20811384; http://dx.doi.org/10.1038/nrd3229 - DOI - PubMed
-
- Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol 2005; 23:1126-36; PMID:16151406; http://dx.doi.org/10.1038/nbt1142 - DOI - PubMed
-
- Nelson AL, Reichert JM. Development trends for therapeutic antibody fragments. Nat Biotechnol 2009; 27:331-7; PMID:19352366; http://dx.doi.org/10.1038/nbt0409-331 - DOI - PubMed
-
- Chen W, Gong R, Ying T, Prabakaran P, Zhu Z, Feng Y, Dimitrov DS. Discovery of novel candidate therapeutics and diagnostics based on engineered human antibody domains. Curr Drug Discov Technol 2014; 11:28-40; PMID:23863097; http://dx.doi.org/10.2174/15701638113109990032 - DOI - PMC - PubMed
-
- Gaberc-Porekar V, Zore I, Podobnik B, Menart V. Obstacles and pitfalls in the PEGylation of therapeutic proteins. Curr Opin Drug Discov Dev 2008; 11:242-50; PMID:18283612 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
