Identification of novel antiacetylated vimentin antibodies in patients with early inflammatory arthritis
- PMID: 26160441
- PMCID: PMC4893102
- DOI: 10.1136/annrheumdis-2014-206785
Identification of novel antiacetylated vimentin antibodies in patients with early inflammatory arthritis
Abstract
Objective: To investigate serum antibody reactivity against a panel of post-translationally modified vimentin peptides (PTMPs) in patients with early inflammatory arthritis.
Methods: A panel of PTMPs was developed. Microtitre plates were coated with peptides derived from vimentin that were identical in length and composition except at one amino acid that was changed to introduce one of three post-translational modifications (PTMs)-either a citrullinated, carbamylated or acetylated residue. Sera of 268 treatment-naive patients with early inflammatory arthritis and symptoms ≤3 months' duration were tested. Patients were assigned to one of three outcome categories at 18-month follow-up (rheumatoid arthritis (RA), persistent non-RA arthritis and resolving arthritis).
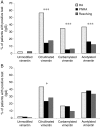
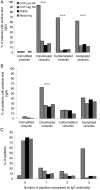
Results: Antibodies against citrullinated, carbamylated and acetylated vimentin peptides were detected in the sera of patients with early inflammatory arthritis. The proportion of patients seropositive for all antibody types was significantly higher in the RA group than in the other groups. Anti cyclic citrullinated peptide (CCP)-positive patients with RA had higher numbers of peptides recognised and higher levels of antibodies against those peptides, representing a distinct profile compared with the other groups.
Conclusions: We show for the first time that antibodies against acetylated vimentin are present in the sera of patients with early RA and confirm and extend previous observations regarding anticitrullinated and anticarbamylated antibodies.
Keywords: Autoantibodies; Early Rheumatoid Arthritis; Rheumatoid Arthritis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures


Similar articles
-
Anti-carbamylated Protein Antibody Levels Correlate with Anti-Sa (Citrullinated Vimentin) Antibody Levels in Rheumatoid Arthritis.J Rheumatol. 2016 Feb;43(2):273-281. doi: 10.3899/jrheum.150179. Epub 2015 Dec 15. J Rheumatol. 2016. PMID: 26669911 Free PMC article.
-
Comparative analysis of autoantibodies targeting peptidylarginine deiminase type 4, mutated citrullinated vimentin and cyclic citrullinated peptides in rheumatoid arthritis: associations with cytokine profiles, clinical and genetic features.Clin Exp Immunol. 2015 Nov;182(2):119-31. doi: 10.1111/cei.12677. Epub 2015 Sep 16. Clin Exp Immunol. 2015. PMID: 26149185 Free PMC article.
-
Selected cyclic citrullinated peptides derived from the sequence of mutated and citrullinated vimentin (MCV) are targeted by different antibodies subclasses in patients with rheumatoid arthritis in Russian patients.Clin Exp Rheumatol. 2014 Sep-Oct;32(5):622-9. Epub 2014 Sep 5. Clin Exp Rheumatol. 2014. PMID: 25189876
-
Novel autoantibodies in rheumatoid arthritis.Reumatismo. 2019 Apr 1;71(1):1-12. doi: 10.4081/reumatismo.2019.1102. Reumatismo. 2019. PMID: 30932437 Review.
-
Anti-citrullinated protein antibodies and their clinical utility in rheumatoid arthritis.Int J Rheum Dis. 2013 Aug;16(4):379-86. doi: 10.1111/1756-185X.12129. Epub 2013 Jul 15. Int J Rheum Dis. 2013. PMID: 23992255 Review.
Cited by
-
The Contribution of Macrophage Plasticity to Inflammatory Arthritis and Their Potential as Therapeutic Targets.Cells. 2024 Sep 20;13(18):1586. doi: 10.3390/cells13181586. Cells. 2024. PMID: 39329767 Free PMC article. Review.
-
Autoantibodies in rheumatoid arthritis - rheumatoid factor, anticitrullinated protein antibodies and beyond.Curr Opin Rheumatol. 2024 May 1;36(3):217-224. doi: 10.1097/BOR.0000000000001006. Epub 2024 Feb 26. Curr Opin Rheumatol. 2024. PMID: 38411194 Free PMC article. Review.
-
Geo-epidemiology of autoantibodies in rheumatoid arthritis: comparison between four ethnically diverse populations.Arthritis Res Ther. 2023 Mar 8;25(1):37. doi: 10.1186/s13075-023-03009-7. Arthritis Res Ther. 2023. PMID: 36890568 Free PMC article.
-
Systemic complications of rheumatoid arthritis: Focus on pathogenesis and treatment.Front Immunol. 2022 Dec 22;13:1051082. doi: 10.3389/fimmu.2022.1051082. eCollection 2022. Front Immunol. 2022. PMID: 36618407 Free PMC article. Review.
-
Implications of Post-Translational Modifications in Autoimmunity with Emphasis on Citrullination, Homocitrullination and Acetylation for the Pathogenesis, Diagnosis and Prognosis of Rheumatoid Arthritis.Int J Mol Sci. 2022 Dec 13;23(24):15803. doi: 10.3390/ijms232415803. Int J Mol Sci. 2022. PMID: 36555449 Free PMC article. Review.
References
-
- Willemze A, Toes RE, Huizinga TW, et al. . New biomarkers in rheumatoid arthritis. Neth J Med 2012;70:392–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
