The Synergistic Effect of Combination Progesterone and Temozolomide on Human Glioblastoma Cells
- PMID: 26110872
- PMCID: PMC4482510
- DOI: 10.1371/journal.pone.0131441
The Synergistic Effect of Combination Progesterone and Temozolomide on Human Glioblastoma Cells
Abstract
Glioblastoma multiforme (GBM) is the most common and most aggressive malignant brain tumor. Despite optimal treatment and evolving standard of care, the median survival of patients diagnosed with GBM is only 12-15 months. In this study, we combined progesterone (PROG) and temozolomide (TMZ), a standard chemotherapeutic agent for human GBM, to test whether PROG enhances the antitumor effects of TMZ and reduces its side effects. Two WHO grade IV human GBM cells lines (U87MG and U118MG) and primary human dermal fibroblasts (HDFs) were repeatedly exposed to PROG and TMZ either alone or in combination for 3 and 6 days. Cell death was measured by MTT reduction assay. PROG and TMZ individually induced tumor cell death in a dose-dependent manner. PROG at high doses produced more cell death than TMZ alone. When combined, PROG enhanced the cell death-inducing effect of TMZ. In HDFs, PROG did not reduce viability even at the same high cytotoxic doses, but TMZ did so in a dose-dependent manner. In combination, PROG reduced TMZ toxicity in HDFs. PROG alone and in combination with TMZ suppressed the EGFR/PI3K/Akt/mTOR signaling pathway and MGMT expression in U87MG cells, thus suppressing cell proliferation. PROG and TMZ individually reduced cell migration in U87MG cells but did so more effectively in combination. PROG enhances the cytotoxic effects of TMZ in GBM cells and reduces its toxic side effects in healthy primary cells.
Conflict of interest statement
Figures
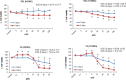
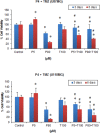
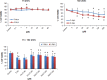


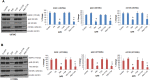
Similar articles
-
NVP-BEZ235, a novel dual PI3K-mTOR inhibitor displays anti-glioma activity and reduces chemoresistance to temozolomide in human glioma cells.Cancer Lett. 2015 Oct 10;367(1):58-68. doi: 10.1016/j.canlet.2015.07.007. Epub 2015 Jul 15. Cancer Lett. 2015. PMID: 26188279
-
Riluzole enhances the antitumor effects of temozolomide via suppression of MGMT expression in glioblastoma.J Neurosurg. 2020 Mar 13;134(3):701-710. doi: 10.3171/2019.12.JNS192682. Print 2021 Mar 1. J Neurosurg. 2020. PMID: 32168477
-
Dual mTORC1/2 blockade inhibits glioblastoma brain tumor initiating cells in vitro and in vivo and synergizes with temozolomide to increase orthotopic xenograft survival.Clin Cancer Res. 2014 Nov 15;20(22):5756-67. doi: 10.1158/1078-0432.CCR-13-3389. Epub 2014 Oct 14. Clin Cancer Res. 2014. PMID: 25316808
-
Recent advances in the use of PI3K inhibitors for glioblastoma multiforme: current preclinical and clinical development.Mol Cancer. 2017 Jun 7;16(1):100. doi: 10.1186/s12943-017-0670-3. Mol Cancer. 2017. PMID: 28592260 Free PMC article. Review.
-
An Interplay between Senescence, Apoptosis and Autophagy in Glioblastoma Multiforme-Role in Pathogenesis and Therapeutic Perspective.Int J Mol Sci. 2018 Mar 17;19(3):889. doi: 10.3390/ijms19030889. Int J Mol Sci. 2018. PMID: 29562589 Free PMC article. Review.
Cited by
-
Proliferative and Invasive Effects of Progesterone-Induced Blocking Factor in Human Glioblastoma Cells.Biomed Res Int. 2017;2017:1295087. doi: 10.1155/2017/1295087. Epub 2017 Jan 12. Biomed Res Int. 2017. PMID: 28168193 Free PMC article.
-
Progesterone at high doses reduces the growth of U87 and A172 glioblastoma cells: Proteomic changes regarding metabolism and immunity.Cancer Med. 2020 Aug;9(16):5767-5780. doi: 10.1002/cam4.3223. Epub 2020 Jun 26. Cancer Med. 2020. PMID: 32590878 Free PMC article.
-
Progesterone Treatment Attenuates Glycolytic Metabolism and Induces Senescence in Glioblastoma.Sci Rep. 2019 Jan 30;9(1):988. doi: 10.1038/s41598-018-37399-5. Sci Rep. 2019. PMID: 30700763 Free PMC article.
-
Progesterone boosts abiraterone-driven target and NK cell therapies against glioblastoma.J Exp Clin Cancer Res. 2024 Aug 6;43(1):218. doi: 10.1186/s13046-024-03144-2. J Exp Clin Cancer Res. 2024. PMID: 39103871 Free PMC article.
-
Impact of sex in the prevalence and progression of glioblastomas: the role of gonadal steroid hormones.Biol Sex Differ. 2021 Mar 22;12(1):28. doi: 10.1186/s13293-021-00372-5. Biol Sex Differ. 2021. PMID: 33752729 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352: 987–996. - PubMed
-
- Ochs K, Kaina B. Apoptosis induced by DNA damage O6-methylguanine is Bcl-2 and caspase-9/3 regulated and Fas/caspase-8 independent. Cancer Res. 2000;60: 5815–5824. - PubMed
-
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352: 997–1003. - PubMed
-
- Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26: 4189–4199. 10.1200/JCO.2007.11.5964 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

