Fat-Specific Protein 27/CIDEC Promotes Development of Alcoholic Steatohepatitis in Mice and Humans
- PMID: 26099526
- PMCID: PMC4584194
- DOI: 10.1053/j.gastro.2015.06.009
Fat-Specific Protein 27/CIDEC Promotes Development of Alcoholic Steatohepatitis in Mice and Humans
Abstract
Background & aims: Alcoholic steatohepatitis (ASH) is the progressive form of alcoholic liver disease and may lead to cirrhosis and hepatocellular carcinoma. We studied mouse models and human tissues to identify molecules associated with ASH progression and focused on the mouse fat-specific protein 27 (FSP-27)/human cell death-inducing DFF45-like effector C (CIDEC) protein, which is expressed in white adipose tissues and promotes formation of fat droplets.
Methods: C57BL/6N mice or mice with hepatocyte-specific disruption of Fsp27 (Fsp27(Hep-/-) mice) were fed the Lieber-Decarli ethanol liquid diet (5% ethanol) for 10 days to 12 weeks, followed by 1 or multiple binges of ethanol (5 or 6 g/kg) during the chronic feeding. Some mice were given an inhibitor (GW9662) of peroxisome proliferator-activated receptor γ (PPARG). Adenoviral vectors were used to express transgenes or small hairpin (sh) RNAs in cultured hepatocytes and in mice. Liver tissue samples were collected from ethanol-fed mice or from 31 patients with alcoholic hepatitis (AH) with biopsy-proved ASH and analyzed histologically and immunohistochemically and by transcriptome, immunoblotting, and real-time PCR analyses.
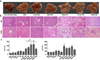
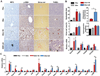
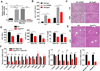
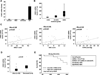
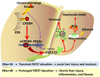
Results: Chronic-plus-binge ethanol feeding of mice, which mimics the drinking pattern of patients with AH, produced severe ASH and mild fibrosis. Microarray analyses revealed similar alterations in expression of many hepatic genes in ethanol-fed mice and humans with ASH, including up-regulation of mouse Fsp27 (also called Cidec) and human CIDEC. Fsp27(Hep-/-) mice and mice given injections of adenovirus-Fsp27shRNA had markedly reduced ASH following chronic-plus-binge ethanol feeding. Inhibition of PPARG and cyclic AMP-responsive element binding protein H (CREBH) prevented the increases in Fsp27α and FSP27β mRNAs, respectively, and reduced liver injury in this chronic-plus-binge ethanol feeding model. Overexpression of FSP27 and ethanol exposure had synergistic effects in inducing production of mitochondrial reactive oxygen species and damage to hepatocytes in mice. Hepatic CIDEC mRNA expression was increased in patients with AH and correlated with the degree of hepatic steatosis and disease severity including mortality.
Conclusions: In mice, chronic-plus-binge ethanol feeding induces ASH that mimics some histological and molecular features observed in patients with AH. Hepatic expression of FSP27/CIDEC is highly up-regulated in mice following chronic-plus-binge ethanol feeding and in patients with AH; this up-regulation contributes to alcohol-induced liver damage.
Keywords: Endoplasmic Reticulum; Inflammation; Lipid Metabolism; Translational Research.
Published by Elsevier Inc.
Conflict of interest statement
Figures


Similar articles
-
Inflammation is independent of steatosis in a murine model of steatohepatitis.Hepatology. 2017 Jul;66(1):108-123. doi: 10.1002/hep.29129. Epub 2017 May 18. Hepatology. 2017. PMID: 28220523 Free PMC article.
-
Pgc1a is responsible for the sex differences in hepatic Cidec/Fsp27β mRNA expression in hepatic steatosis of mice fed a Western diet.Am J Physiol Endocrinol Metab. 2020 Feb 1;318(2):E249-E261. doi: 10.1152/ajpendo.00199.2019. Epub 2019 Dec 17. Am J Physiol Endocrinol Metab. 2020. PMID: 31846369
-
Luteolin alleviates alcoholic liver disease induced by chronic and binge ethanol feeding in mice.J Nutr. 2014 Jul;144(7):1009-15. doi: 10.3945/jn.114.193128. Epub 2014 May 14. J Nutr. 2014. PMID: 24828027
-
A physiological role for fat specific protein 27/cell death-inducing DFF45-like effector C in adipose and liver.Biol Pharm Bull. 2010;33(3):346-50. doi: 10.1248/bpb.33.346. Biol Pharm Bull. 2010. PMID: 20190390 Review.
-
Obesity and binge alcohol intake are deadly combination to induce steatohepatitis: A model of high-fat diet and binge ethanol intake.Clin Mol Hepatol. 2020 Oct;26(4):586-594. doi: 10.3350/cmh.2020.0100. Epub 2020 Sep 17. Clin Mol Hepatol. 2020. PMID: 32937687 Free PMC article. Review.
Cited by
-
Alcohol-associated liver disease.J Clin Invest. 2024 Feb 1;134(3):e176345. doi: 10.1172/JCI176345. J Clin Invest. 2024. PMID: 38299591 Free PMC article. Review.
-
Disruption of hepatic small heterodimer partner induces dissociation of steatosis and inflammation in experimental nonalcoholic steatohepatitis.J Biol Chem. 2020 Jan 24;295(4):994-1008. doi: 10.1074/jbc.RA119.010233. Epub 2019 Dec 12. J Biol Chem. 2020. PMID: 31831621 Free PMC article.
-
Hairy and enhancer of split 6 prevents hepatic lipid accumulation through inhibition of Pparg2 expression.Hepatol Commun. 2017 Nov 8;1(10):1085-1098. doi: 10.1002/hep4.1120. eCollection 2017 Dec. Hepatol Commun. 2017. PMID: 29404444 Free PMC article.
-
CircRNA expression pattern and circRNA-miRNA-mRNA network in the pathogenesis of nonalcoholic steatohepatitis.Oncotarget. 2016 Oct 11;7(41):66455-66467. doi: 10.18632/oncotarget.12186. Oncotarget. 2016. PMID: 27677588 Free PMC article.
-
High-fat diet induced obesity promotes inflammation, oxidative stress, and hepatotoxicity in female FVB/N mice.Biofactors. 2024 May-Jun;50(3):572-591. doi: 10.1002/biof.2028. Epub 2024 Jan 6. Biofactors. 2024. PMID: 38183321
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

