Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells
- PMID: 26097885
- PMCID: PMC4468338
- DOI: 10.18632/oncoscience.160
Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells
Abstract
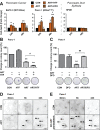
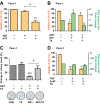
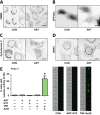
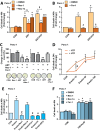
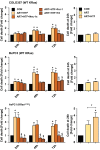
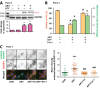
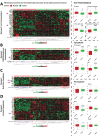
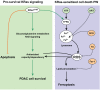
Oncogenic KRas reprograms pancreatic ductal adenocarcinoma (PDAC) cells to states which are highly resistant to apoptosis. Thus, a major preclinical goal is to identify effective strategies for killing PDAC cells. Artesunate (ART) is an anti-malarial that specifically induces programmed cell death in different cancer cell types, in a manner initiated by reactive oxygen species (ROS)-generation. In this study we demonstrate that ART specifically induced ROS- and lysosomal iron-dependent cell death in PDAC cell lines. Highest cytotoxicity was obtained in PDAC cell lines with constitutively-active KRas, and ART did not affect non-neoplastic human pancreatic ductal epithelial (HPDE) cells. We determined that ART did not induce apoptosis or necroptosis. Instead, ART induced ferroptosis, a recently described mode of ROS- and iron-dependent programmed necrosis which can be activated in Ras-transformed cells. Co-treatment with the ferroptosis inhibitor ferrostatin-1 blocked ART-induced lipid peroxidation and cell death, and increased long-term cell survival and proliferation. Importantly, analysis of PDAC patient mRNA expression indicates a dependency on antioxidant homeostasis and increased sensitivity to free intracellular iron, both of which correlate with Ras-driven sensitivity to ferroptosis. Overall, our findings suggest that ART activation of ferroptosis is an effective, novel pathway for killing PDAC cells.
Keywords: KRas; artesunate; cell death; ferroptosis; necroptosis; pancreatic cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Role of GRP78 inhibiting artesunate-induced ferroptosis in KRAS mutant pancreatic cancer cells.Drug Des Devel Ther. 2019 Jul 2;13:2135-2144. doi: 10.2147/DDDT.S199459. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31456633 Free PMC article.
-
Artesunate induces ferroptosis via modulation of p38 and ERK signaling pathway in glioblastoma cells.J Pharmacol Sci. 2022 Mar;148(3):300-306. doi: 10.1016/j.jphs.2022.01.007. Epub 2022 Jan 13. J Pharmacol Sci. 2022. PMID: 35177209
-
The Art of War: Ferroptosis and Pancreatic Cancer.Front Pharmacol. 2021 Dec 10;12:773909. doi: 10.3389/fphar.2021.773909. eCollection 2021. Front Pharmacol. 2021. PMID: 34955844 Free PMC article. Review.
-
Ferroptosis: New Strategies and Ideas for the Treatment of Pancreatic Ductal Adenocarcinoma.Front Biosci (Landmark Ed). 2024 Jan 23;29(1):45. doi: 10.31083/j.fbl2901045. Front Biosci (Landmark Ed). 2024. PMID: 38287825 Review.
-
Phosphorescent rhenium(I) complexes conjugated with artesunate: Mitochondrial targeting and apoptosis-ferroptosis dual induction.J Inorg Biochem. 2021 Oct;223:111537. doi: 10.1016/j.jinorgbio.2021.111537. Epub 2021 Jul 9. J Inorg Biochem. 2021. PMID: 34273716
Cited by
-
Ferroptosis: Biochemistry and Biology in Cancers.Front Oncol. 2021 Apr 1;11:579286. doi: 10.3389/fonc.2021.579286. eCollection 2021. Front Oncol. 2021. PMID: 33868986 Free PMC article. Review.
-
Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research.J Hematol Oncol. 2022 Dec 8;15(1):174. doi: 10.1186/s13045-022-01392-3. J Hematol Oncol. 2022. PMID: 36482419 Free PMC article. Review.
-
Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine.Chin Med. 2019 Nov 6;14:48. doi: 10.1186/s13020-019-0270-9. eCollection 2019. Chin Med. 2019. PMID: 31719837 Free PMC article. Review.
-
YM155, specific survivin inhibitor, can enhance artesunate-induced cytotoxicity in HCT116 colon cancer cells.Korean J Clin Oncol. 2020 Dec;16(2):131-137. doi: 10.14216/kjco.20020. Epub 2020 Dec 31. Korean J Clin Oncol. 2020. PMID: 36945719 Free PMC article.
-
Ferroptosis and Its Role in Chronic Diseases.Cells. 2022 Jun 27;11(13):2040. doi: 10.3390/cells11132040. Cells. 2022. PMID: 35805124 Free PMC article. Review.
References
-
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. - PubMed
-
- Malvezzi M, Bertuccio P, Levi F, La Vecchia C Negri E. European cancer mortality predictions for the year. Ann Oncol. 2014:2014. - PubMed
-
- Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53(4):549–554. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

