The MicroRNA-132 and MicroRNA-212 Cluster Regulates Hematopoietic Stem Cell Maintenance and Survival with Age by Buffering FOXO3 Expression
- PMID: 26084022
- PMCID: PMC4471877
- DOI: 10.1016/j.immuni.2015.05.017
The MicroRNA-132 and MicroRNA-212 Cluster Regulates Hematopoietic Stem Cell Maintenance and Survival with Age by Buffering FOXO3 Expression
Abstract
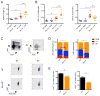
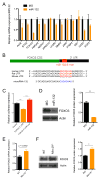
MicroRNAs are critical post-transcriptional regulators of hematopoietic cell-fate decisions, though little remains known about their role in aging hematopoietic stem cells (HSCs). We found that the microRNA-212/132 cluster (Mirc19) is enriched in HSCs and is upregulated during aging. Both overexpression and deletion of microRNAs in this cluster leads to inappropriate hematopoiesis with age. Enforced expression of miR-132 in the bone marrow of mice led to rapid HSC cycling and depletion. A genetic deletion of Mirc19 in mice resulted in HSCs that had altered cycling, function, and survival in response to growth factor starvation. We found that miR-132 exerted its effect on aging HSCs by targeting the transcription factor FOXO3, a known aging associated gene. Our data demonstrate that Mirc19 plays a role in maintaining balanced hematopoietic output by buffering FOXO3 expression. We have thus identified it as a potential target that might play a role in age-related hematopoietic defects.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
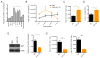

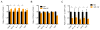
Similar articles
-
GABP controls a critical transcription regulatory module that is essential for maintenance and differentiation of hematopoietic stem/progenitor cells.Blood. 2011 Feb 17;117(7):2166-78. doi: 10.1182/blood-2010-09-306563. Epub 2010 Dec 7. Blood. 2011. PMID: 21139080 Free PMC article.
-
Inactivation of the forkhead transcription factor FoxO3 is essential for PKB-mediated survival of hematopoietic progenitor cells by kit ligand.Exp Hematol. 2003 Apr;31(4):316-23. doi: 10.1016/s0301-472x(03)00002-x. Exp Hematol. 2003. PMID: 12691919
-
FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress.Cell. 2007 Jan 26;128(2):325-39. doi: 10.1016/j.cell.2007.01.003. Cell. 2007. PMID: 17254970
-
Concise review: Exploring miRNAs--toward a better understanding of hematopoiesis.Stem Cells. 2015 Jan;33(1):1-7. doi: 10.1002/stem.1810. Stem Cells. 2015. PMID: 25132287 Review.
-
microRNAs in hematopoiesis.Exp Cell Res. 2014 Dec 10;329(2):234-8. doi: 10.1016/j.yexcr.2014.08.033. Epub 2014 Sep 3. Exp Cell Res. 2014. PMID: 25192911 Review.
Cited by
-
MicroRNAs-associated with FOXO3 in cellular senescence and other stress responses.Biogerontology. 2024 Feb;25(1):23-51. doi: 10.1007/s10522-023-10059-6. Epub 2023 Aug 30. Biogerontology. 2024. PMID: 37646881 Review.
-
MicroRNA-21 maintains hematopoietic stem cell homeostasis through sustaining the NF-κB signaling pathway in mice.Haematologica. 2021 Feb 1;106(2):412-423. doi: 10.3324/haematol.2019.236927. Haematologica. 2021. PMID: 31974197 Free PMC article.
-
miR-126 Regulates Distinct Self-Renewal Outcomes in Normal and Malignant Hematopoietic Stem Cells.Cancer Cell. 2016 Feb 8;29(2):214-28. doi: 10.1016/j.ccell.2015.12.011. Epub 2016 Jan 28. Cancer Cell. 2016. PMID: 26832662 Free PMC article.
-
Regulation of adult stem cell quiescence and its functions in the maintenance of tissue integrity.Nat Rev Mol Cell Biol. 2023 May;24(5):334-354. doi: 10.1038/s41580-022-00568-6. Epub 2023 Mar 15. Nat Rev Mol Cell Biol. 2023. PMID: 36922629 Free PMC article. Review.
-
Hematopoietic stem cells under pressure.Curr Opin Hematol. 2017 Jul;24(4):314-321. doi: 10.1097/MOH.0000000000000347. Curr Opin Hematol. 2017. PMID: 28375987 Free PMC article. Review.
References
-
- Baltimore D, Boldin MP, O’Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nature immunology. 2008;9:839–845. - PubMed
-
- Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science (New York, NY) 2004;303:83–86. - PubMed
-
- Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nature reviews Molecular cell biology. 2013;14:83–97. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

