A randomized double-blind, placebo-, and active-controlled study of T-type calcium channel blocker ABT-639 in patients with diabetic peripheral neuropathic pain
- PMID: 26067585
- PMCID: PMC4770341
- DOI: 10.1097/j.pain.0000000000000263
A randomized double-blind, placebo-, and active-controlled study of T-type calcium channel blocker ABT-639 in patients with diabetic peripheral neuropathic pain
Abstract
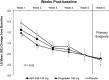
T-type Cav3.2 calcium channels represent a novel target for neuropathic pain modulation. Preclinical studies with ABT-639, a peripherally acting highly selective T-type Cav3.2 calcium channel blocker, showed dose-dependent reduction of pain in multiple pain models. ABT-639 also demonstrated an acceptable safety profile at single- and multiple-dose levels evaluated in a clinical phase 1 study in healthy volunteers. The primary objective of this phase 2, multicenter, randomized, double-blind, placebo-controlled, and active-controlled study was to compare the analgesic efficacy and safety of ABT-639 with placebo in the treatment of diabetic neuropathic pain. Pregabalin, an approved treatment for painful diabetic neuropathy, was included as a positive control. A total of 194 patients were randomized and treated for 6 weeks; 62 patients received ABT-639 (100 mg twice daily), 70 patients received pregabalin (150 mg twice daily), and 62 patients received placebo. When assessing the mean changes from baseline in patient-recorded pain scores at the end of week 6, there was no significant difference observed for ABT-639 compared with placebo (-2.28 vs -2.36; P = 0.582). Pregabalin treatment resulted in a transient improvement in pain compared with placebo, which did not persist throughout the study. There were no significant safety issues identified with ABT-639. A majority of adverse events were considered mild to moderate in intensity. In conclusion, treatment with the highly selective T-type Cav3.2 calcium channel blocker ABT-639 100 mg twice daily for 6 weeks showed no safety signals that would preclude further investigation but did not reduce neuropathic pain in patients with diabetes (ClinicalTrials.gov identifier: NCT01345045).
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures
Similar articles
-
Effects of a T-type calcium channel blocker, ABT-639, on spontaneous activity in C-nociceptors in patients with painful diabetic neuropathy: a randomized controlled trial.Pain. 2015 Nov;156(11):2175-2183. doi: 10.1097/j.pain.0000000000000249. Pain. 2015. PMID: 26035253 Clinical Trial.
-
A Randomized, Double-Blind, Placebo-Controlled, Crossover Study of the T-Type Calcium Channel Blocker ABT-639 in an Intradermal Capsaicin Experimental Pain Model in Healthy Adults.Pain Med. 2016 Mar;17(3):551-560. doi: 10.1093/pm/pnv068. Epub 2015 Dec 16. Pain Med. 2016. PMID: 26814294 Clinical Trial.
-
A peripherally acting, selective T-type calcium channel blocker, ABT-639, effectively reduces nociceptive and neuropathic pain in rats.Biochem Pharmacol. 2014 Jun 15;89(4):536-44. doi: 10.1016/j.bcp.2014.03.015. Epub 2014 Apr 12. Biochem Pharmacol. 2014. PMID: 24726441
-
The effect of pregabalin on pain-related sleep interference in diabetic peripheral neuropathy or postherpetic neuralgia: a review of nine clinical trials.Curr Med Res Opin. 2010 Oct;26(10):2411-9. doi: 10.1185/03007995.2010.516142. Curr Med Res Opin. 2010. PMID: 20812792 Review.
-
Pregabalin: in the treatment of painful diabetic peripheral neuropathy.Drugs. 2004;64(24):2813-20; discussion 2821. doi: 10.2165/00003495-200464240-00006. Drugs. 2004. PMID: 15563250 Review.
Cited by
-
Modulation of T-type Ca2+ channels by Lavender and Rosemary extracts.PLoS One. 2017 Oct 26;12(10):e0186864. doi: 10.1371/journal.pone.0186864. eCollection 2017. PLoS One. 2017. PMID: 29073181 Free PMC article.
-
Ion channels and neuronal hyperexcitability in chemotherapy-induced peripheral neuropathy; cause and effect?Mol Pain. 2017 Jan-Dec;13:1744806917714693. doi: 10.1177/1744806917714693. Mol Pain. 2017. PMID: 28580836 Free PMC article.
-
Betulinic acid analogs inhibit N- and T-type voltage-gated calcium channels to attenuate nerve-injury associated neuropathic and formalin models of pain.Neurobiol Pain. 2023 Jan 14;13:100116. doi: 10.1016/j.ynpai.2023.100116. eCollection 2023 Jan-Jul. Neurobiol Pain. 2023. PMID: 36687466 Free PMC article.
-
Peripheral Voltage-Gated Cation Channels in Neuropathic Pain and Their Potential as Therapeutic Targets.Front Pain Res (Lausanne). 2021 Dec 13;2:750583. doi: 10.3389/fpain.2021.750583. eCollection 2021. Front Pain Res (Lausanne). 2021. PMID: 35295464 Free PMC article. Review.
-
Experimental Drugs for Neuropathic Pain.Curr Neuropharmacol. 2018;16(8):1193-1209. doi: 10.2174/1570159X16666180510151241. Curr Neuropharmacol. 2018. PMID: 29745335 Free PMC article. Review.
References
-
- Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell KP. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science 2003;302:1416–18. - PubMed
-
- Jarvis MF, Scott VE, McGaraughty S, Chu KL, Xu J, Niforatos W, Milicic I, Joshi S, Zhang Q, Xia Z. A peripherally acting, selective T-type calcium channel blocker, ABT-639, effectively reduces nociceptive and neuropathic pain in rats. Biochem Pharmacol 2014;89:536–44. - PubMed
-
- Jarvis MF, Scott VE, McGaraughty S, Niforatos W, Milicic I, Joshi S, Zhang S, Xia Z. A peripherally acting, selective T-type calcium channel blocker, ABT-639, effectively reduces nociceptive and neuropathic pain in rats. Presented at the Society for Neuroscience, November 13, 2013; Poster 829.01. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

