Lower Respiratory Tract Infection of the Ferret by 2009 H1N1 Pandemic Influenza A Virus Triggers Biphasic, Systemic, and Local Recruitment of Neutrophils
- PMID: 26063430
- PMCID: PMC4524093
- DOI: 10.1128/JVI.00817-15
Lower Respiratory Tract Infection of the Ferret by 2009 H1N1 Pandemic Influenza A Virus Triggers Biphasic, Systemic, and Local Recruitment of Neutrophils
Abstract
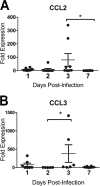
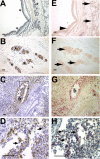
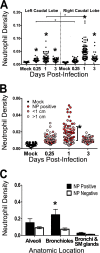
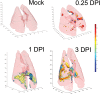
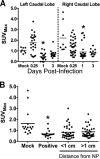
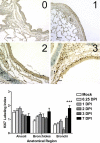
Infection of the lower respiratory tract by influenza A viruses results in increases in inflammation and immune cell infiltration in the lung. The dynamic relationships among the lung microenvironments, the lung, and systemic host responses during infection remain poorly understood. Here we used extensive systematic histological analysis coupled with live imaging to gain access to these relationships in ferrets infected with the 2009 H1N1 pandemic influenza A virus (H1N1pdm virus). Neutrophil levels rose in the lungs of H1N1pdm virus-infected ferrets 6 h postinfection and became concentrated at areas of the H1N1pdm virus-infected bronchiolar epithelium by 1 day postinfection (dpi). In addition, neutrophil levels were increased throughout the alveolar spaces during the first 3 dpi and returned to baseline by 6 dpi. Histochemical staining revealed that neutrophil infiltration in the lungs occurred in two waves, at 1 and 3 dpi, and gene expression within microenvironments suggested two types of neutrophils. Specifically, CCL3 levels, but not CXCL8/interleukin 8 (IL-8) levels, were higher within discrete lung microenvironments and coincided with increased infiltration of neutrophils into the lung. We used live imaging of ferrets to monitor host responses within the lung over time with [(18)F]fluorodeoxyglucose (FDG). Sites in the H1N1pdm virus-infected ferret lung with high FDG uptake had high levels of proliferative epithelium. In summary, neutrophils invaded the H1N1pdm virus-infected ferret lung globally and focally at sites of infection. Increased neutrophil levels in microenvironments did not correlate with increased FDG uptake; hence, FDG uptake may reflect prior infection and inflammation of lungs that have experienced damage, as evidenced by bronchial regeneration of tissues in the lungs at sites with high FDG levels.
Importance: Severe influenza disease is characterized by an acute infection of the lower airways that may progress rapidly to organ failure and death. Well-developed animal models that mimic human disease are essential to understanding the complex relationships of the microenvironment, organ, and system in controlling virus replication, inflammation, and disease progression. Employing the ferret model of H1N1pdm virus infection, we used live imaging and comprehensive histological analyses to address specific hypotheses regarding spatial and temporal relationships that occur during the progression of infection and inflammation. We show the general invasion of neutrophils at the organ level (lung) but also a distinct pattern of localized accumulation within the microenvironment at the site of infection. Moreover, we show that these responses were biphasic within the lung. Finally, live imaging revealed an early and sustained host metabolic response at sites of infection that may reflect damage and repair of tissues in the lungs.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Molecular imaging reveals a progressive pulmonary inflammation in lower airways in ferrets infected with 2009 H1N1 pandemic influenza virus.PLoS One. 2012;7(7):e40094. doi: 10.1371/journal.pone.0040094. Epub 2012 Jul 20. PLoS One. 2012. PMID: 22911695 Free PMC article.
-
The severe pathogenicity of alveolar macrophage-depleted ferrets infected with 2009 pandemic H1N1 influenza virus.Virology. 2013 Sep;444(1-2):394-403. doi: 10.1016/j.virol.2013.07.006. Epub 2013 Jul 23. Virology. 2013. PMID: 23890814
-
H7N9 Influenza Virus Is More Virulent in Ferrets than 2009 Pandemic H1N1 Influenza Virus.Viral Immunol. 2015 Dec;28(10):590-9. doi: 10.1089/vim.2015.0052. Epub 2015 Sep 30. Viral Immunol. 2015. PMID: 26421365
-
The Intersection of Age and Influenza Severity: Utility of Ferrets for Dissecting the Age-Dependent Immune Responses and Relevance to Age-Specific Vaccine Development.Viruses. 2021 Apr 15;13(4):678. doi: 10.3390/v13040678. Viruses. 2021. PMID: 33920917 Free PMC article. Review.
-
Innate Immune Responses to Influenza Virus Infections in the Upper Respiratory Tract.Viruses. 2021 Oct 17;13(10):2090. doi: 10.3390/v13102090. Viruses. 2021. PMID: 34696520 Free PMC article. Review.
Cited by
-
Extracellular Traps: An Ancient Weapon of Multiple Kingdoms.Biology (Basel). 2020 Feb 18;9(2):34. doi: 10.3390/biology9020034. Biology (Basel). 2020. PMID: 32085405 Free PMC article. Review.
-
Manipulating the air-filled zebrafish swim bladder as a neutrophilic inflammation model for acute lung injury.Cell Death Dis. 2016 Nov 10;7(11):e2470. doi: 10.1038/cddis.2016.365. Cell Death Dis. 2016. PMID: 27831560 Free PMC article.
-
A Role for Neutrophils in Viral Respiratory Disease.Front Immunol. 2017 May 12;8:550. doi: 10.3389/fimmu.2017.00550. eCollection 2017. Front Immunol. 2017. PMID: 28553293 Free PMC article. Review.
-
Immunopathological similarities between COVID-19 and influenza: Investigating the consequences of Co-infection.Microb Pathog. 2021 Mar;152:104554. doi: 10.1016/j.micpath.2020.104554. Epub 2020 Nov 3. Microb Pathog. 2021. PMID: 33157216 Free PMC article. Review.
-
Age-Related Pathology Associated with H1N1 A/California/07/2009 Influenza Virus Infection.Am J Pathol. 2019 Dec;189(12):2389-2399. doi: 10.1016/j.ajpath.2019.08.017. Epub 2019 Oct 1. Am J Pathol. 2019. PMID: 31585069 Free PMC article.
References
-
- Preaud E, Durand L, Macabeo B, Farkas N, Sloesen B, Palache A, Shupo F, Samson SI, Vaccines Europe influenza working group. 2014. Annual public health and economic benefits of seasonal influenza vaccination: a European estimate. BMC Public Health 14:813. doi:10.1186/1471-2458-14-813. - DOI - PMC - PubMed
-
- Lu PJ, Santibanez TA, Williams WW, Zhang J, Ding H, Bryan L, O'Halloran A, Greby SM, Bridges CB, Graitcer SB, Kennedy ED, Lindley MC, Ahluwalia IB, LaVail K, Pabst LJ, Harris L, Vogt T, Town M, Singleton JA, Centers for Disease Control and Prevention. 2013. Surveillance of influenza vaccination coverage—United States, 2007–08 through 2011–12 influenza seasons. MMWR Surveill Summ 62(SS-04):1–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

