Viral apoptotic mimicry
- PMID: 26052667
- PMCID: PMC7097103
- DOI: 10.1038/nrmicro3469
Viral apoptotic mimicry
Abstract
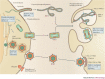
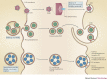
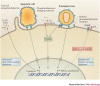
As opportunistic pathogens, viruses have evolved many elegant strategies to manipulate host cells for infectious entry and replication. Viral apoptotic mimicry, defined by the exposure of phosphatidylserine - a marker for apoptosis - on the pathogen surface, is emerging as a common theme used by enveloped viruses to promote infection. Focusing on the four best described examples (vaccinia virus, dengue virus, Ebola virus and pseudotyped lentivirus), we summarize our current understanding of apoptotic mimicry as a mechanism for virus entry, binding and immune evasion. We also describe recent examples of non-enveloped viruses that use this mimicry strategy, and discuss future directions and how viral apoptotic mimicry could be targeted therapeutically.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Role of phosphatidylserine receptors in enveloped virus infection.J Virol. 2014 Apr;88(8):4275-90. doi: 10.1128/JVI.03287-13. Epub 2014 Jan 29. J Virol. 2014. PMID: 24478428 Free PMC article.
-
mSphere of Influence: Apoptotic Mimicry and Virus Entry.mSphere. 2021 Jan 27;6(1):e00034-21. doi: 10.1128/mSphere.00034-21. mSphere. 2021. PMID: 33504657 Free PMC article.
-
Exploitation of glycosylation in enveloped virus pathobiology.Biochim Biophys Acta Gen Subj. 2019 Oct;1863(10):1480-1497. doi: 10.1016/j.bbagen.2019.05.012. Epub 2019 May 20. Biochim Biophys Acta Gen Subj. 2019. PMID: 31121217 Free PMC article. Review.
-
Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells.Science. 2008 Apr 25;320(5875):531-5. doi: 10.1126/science.1155164. Science. 2008. PMID: 18436786
-
Molecular Mechanism of Externalization of Phosphatidylserine on the Surface of Ebola Virus Particles.DNA Cell Biol. 2019 Feb;38(2):115-120. doi: 10.1089/dna.2018.4485. Epub 2019 Jan 7. DNA Cell Biol. 2019. PMID: 30615471 Review.
Cited by
-
TAM Receptor Inhibition-Implications for Cancer and the Immune System.Cancers (Basel). 2021 Mar 10;13(6):1195. doi: 10.3390/cancers13061195. Cancers (Basel). 2021. PMID: 33801886 Free PMC article. Review.
-
The board is set, the pieces are moving: Modulation of New World arenavirus entry by host proteins.PLoS Pathog. 2021 Jun 10;17(6):e1009605. doi: 10.1371/journal.ppat.1009605. eCollection 2021 Jun. PLoS Pathog. 2021. PMID: 34111222 Free PMC article. Review. No abstract available.
-
The Role of TIM-1 and CD300a in Zika Virus Infection Investigated with Cell-Based Electrical Impedance.Biosensors (Basel). 2024 Jul 25;14(8):362. doi: 10.3390/bios14080362. Biosensors (Basel). 2024. PMID: 39194591 Free PMC article.
-
Enterovirus Transmission by Secretory Autophagy.Viruses. 2018 Mar 20;10(3):139. doi: 10.3390/v10030139. Viruses. 2018. PMID: 29558400 Free PMC article. Review.
-
Extracellular vesicles block viral entryways.Nat Microbiol. 2024 Apr;9(4):882-883. doi: 10.1038/s41564-024-01651-8. Nat Microbiol. 2024. PMID: 38528149 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

