Inhibition of TRPM7 by carvacrol suppresses glioblastoma cell proliferation, migration and invasion
- PMID: 25965832
- PMCID: PMC4599272
- DOI: 10.18632/oncotarget.3872
Inhibition of TRPM7 by carvacrol suppresses glioblastoma cell proliferation, migration and invasion
Abstract
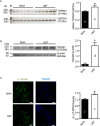
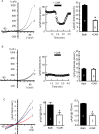
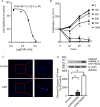
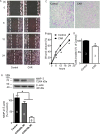
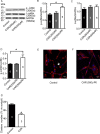
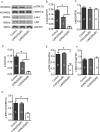
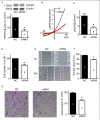
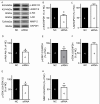
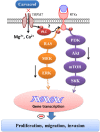
Glioblastomas are progressive brain tumors with devastating proliferative and invasive characteristics. Ion channels are the second largest target class for drug development. In this study, we investigated the effects of the TRPM7 inhibitor carvacrol on the viability, resistance to apoptosis, migration, and invasiveness of the human U87 glioblastoma cell line.The expression levels of TRPM7 mRNA and protein in U87 cells were detected by RT-PCR, western blotting and immunofluorescence. TRPM7 currents were recorded using whole-cell patch-clamp techniques. An MTT assay was used to assess cell viability and proliferation. Wound healing and transwell experiments were used to evaluate cell migration and invasion. Protein levels of p-Akt/t-Akt, p-ERK1/2/t-ERK1/2, cleaved caspase-3, MMP-2 and phosphorylated cofilin were also detected.TRPM7 mRNA and protein expression in U87 cells is higher than in normal human astrocytes. Whole-cell patch-clamp recording showed that carvacrol blocks recombinant TRPM7 current in HEK293 cells and endogenous TRPM7-like current in U87 cells. Carvacrol treatment reduced the viability, migration and invasion of U87 cells. Carvacrol also decreased MMP-2 protein expression and promoted the phosphorylation of cofilin. Furthermore, carvacrol inhibited the Ras/MEK/MAPK and PI3K/Akt signaling pathways.Therefore, carvacrol may have therapeutic potential for the treatment of glioblastomas through its inhibition of TRPM7 channels.
Keywords: TRPM7; carvacrol; cell viability; glioblastoma; invasion; migration.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Xyloketal B suppresses glioblastoma cell proliferation and migration in vitro through inhibiting TRPM7-regulated PI3K/Akt and MEK/ERK signaling pathways.Mar Drugs. 2015 Apr 22;13(4):2505-25. doi: 10.3390/md13042505. Mar Drugs. 2015. PMID: 25913706 Free PMC article.
-
Activation of TRPM7 by naltriben enhances migration and invasion of glioblastoma cells.Oncotarget. 2017 Feb 14;8(7):11239-11248. doi: 10.18632/oncotarget.14496. Oncotarget. 2017. PMID: 28061441 Free PMC article.
-
Carvacrol Alleviates Prostate Cancer Cell Proliferation, Migration, and Invasion through Regulation of PI3K/Akt and MAPK Signaling Pathways.Oxid Med Cell Longev. 2016;2016:1469693. doi: 10.1155/2016/1469693. Epub 2016 Oct 10. Oxid Med Cell Longev. 2016. PMID: 27803760 Free PMC article.
-
Modulators of TRPM7 and its potential as a drug target for brain tumours.Cell Calcium. 2022 Jan;101:102521. doi: 10.1016/j.ceca.2021.102521. Epub 2021 Dec 17. Cell Calcium. 2022. PMID: 34953296 Review.
-
Oncogenic role and therapeutic target of transient receptor potential melastatin 7 channel in malignancy.Expert Opin Ther Targets. 2014 Oct;18(10):1177-96. doi: 10.1517/14728222.2014.940894. Epub 2014 Jul 29. Expert Opin Ther Targets. 2014. PMID: 25069584 Review.
Cited by
-
Ca2+ as a therapeutic target in cancer.Adv Cancer Res. 2020;148:233-317. doi: 10.1016/bs.acr.2020.05.003. Epub 2020 Jul 9. Adv Cancer Res. 2020. PMID: 32723565 Free PMC article. Review.
-
TRPM7 is overexpressed in bladder cancer and promotes proliferation, migration, invasion and tumor growth.Oncol Rep. 2017 Oct;38(4):1967-1976. doi: 10.3892/or.2017.5883. Epub 2017 Aug 7. Oncol Rep. 2017. PMID: 28791418 Free PMC article.
-
Role of TRPM7 in cerebral ischaemia and hypoxia.J Physiol. 2017 May 15;595(10):3077-3083. doi: 10.1113/JP273709. Epub 2017 Feb 8. J Physiol. 2017. PMID: 27891609 Free PMC article. Review.
-
TRPM7 channel inhibition mediates midazolam-induced proliferation loss in human malignant glioma.Tumour Biol. 2016 Nov;37(11):14721-14731. doi: 10.1007/s13277-016-5317-2. Epub 2016 Sep 14. Tumour Biol. 2016. PMID: 27629139
-
Inhibition of TRPM7 blocks MRTF/SRF-dependent transcriptional and tumorigenic activity.Oncogene. 2020 Mar;39(11):2328-2344. doi: 10.1038/s41388-019-1140-8. Epub 2019 Dec 16. Oncogene. 2020. PMID: 31844251
References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology. 2009;10:459–466. - PubMed
-
- Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352:987–996. - PubMed
-
- Chen J, Yakisich JS. Editorial: emerging concepts and therapeutics strategies for the treatment of brain tumors. Anti-cancer agents in medicinal chemistry. 2014;14:1063–1064. - PubMed
-
- Leon SP, Zhu J, Black PM. Genetic aberrations in human brain tumors. Neurosurgery. 1994;34:708–722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

