TIGIT and PD-1 impair tumor antigen-specific CD8⁺ T cells in melanoma patients
- PMID: 25866972
- PMCID: PMC4463210
- DOI: 10.1172/JCI80445
TIGIT and PD-1 impair tumor antigen-specific CD8⁺ T cells in melanoma patients
Abstract
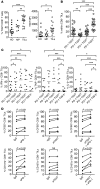
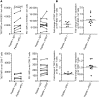
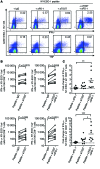
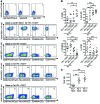
T cell Ig and ITIM domain (TIGIT) is an inhibitory receptor expressed by activated T cells, Tregs, and NK cells. Here, we determined that TIGIT is upregulated on tumor antigen-specific (TA-specific) CD8⁺ T cells and CD8⁺ tumor-infiltrating lymphocytes (TILs) from patients with melanoma, and these TIGIT-expressing CD8⁺ T cells often coexpress the inhibitory receptor PD-1. Moreover, CD8⁺ TILs from patients exhibited downregulation of the costimulatory molecule CD226, which competes with TIGIT for the same ligand, supporting a TIGIT/CD226 imbalance in metastatic melanoma. TIGIT marked early T cell activation and was further upregulated by T cells upon PD-1 blockade and in dysfunctional PD-1⁺TIM-3⁺ TA-specific CD8⁺ T cells. PD-1⁺TIGIT⁺, PD-1⁻TIGIT⁺, and PD-1⁺TIGIT⁻ CD8⁺ TILs had similar functional capacities ex vivo, suggesting that TIGIT alone, or together with PD-1, is not indicative of T cell dysfunction. However, in the presence of TIGIT ligand-expressing cells, TIGIT and PD-1 blockade additively increased proliferation, cytokine production, and degranulation of both TA-specific CD8⁺ T cells and CD8⁺ TILs. Collectively, our results show that TIGIT and PD-1 regulate the expansion and function of TA-specific CD8⁺ T cells and CD8⁺ TILs in melanoma patients and suggest that dual TIGIT and PD-1 blockade should be further explored to elicit potent antitumor CD8⁺ T cell responses in patients with advanced melanoma.
Figures




Similar articles
-
TIGIT and PD1 Co-blockade Restores ex vivo Functions of Human Tumor-Infiltrating CD8+ T Cells in Hepatocellular Carcinoma.Cell Mol Gastroenterol Hepatol. 2021;12(2):443-464. doi: 10.1016/j.jcmgh.2021.03.003. Epub 2021 Mar 27. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33781741 Free PMC article.
-
TOX-expressing terminally exhausted tumor-infiltrating CD8+ T cells are reinvigorated by co-blockade of PD-1 and TIGIT in bladder cancer.Cancer Lett. 2021 Feb 28;499:137-147. doi: 10.1016/j.canlet.2020.11.035. Epub 2020 Nov 27. Cancer Lett. 2021. PMID: 33249194
-
IL15 Stimulation with TIGIT Blockade Reverses CD155-mediated NK-Cell Dysfunction in Melanoma.Clin Cancer Res. 2020 Oct 15;26(20):5520-5533. doi: 10.1158/1078-0432.CCR-20-0575. Epub 2020 Jun 26. Clin Cancer Res. 2020. PMID: 32591463 Free PMC article.
-
TIGIT, A Novel Therapeutic Target for Tumor Immunotherapy.Immunol Invest. 2017 Feb;46(2):172-182. doi: 10.1080/08820139.2016.1237524. Epub 2016 Nov 7. Immunol Invest. 2017. PMID: 27819527 Review.
-
Effector, Memory, and Dysfunctional CD8(+) T Cell Fates in the Antitumor Immune Response.J Immunol Res. 2016;2016:8941260. doi: 10.1155/2016/8941260. Epub 2016 May 22. J Immunol Res. 2016. PMID: 27314056 Free PMC article. Review.
Cited by
-
Next Generation Immuno-Oncology Strategies: Unleashing NK Cells Activity.Cells. 2022 Oct 6;11(19):3147. doi: 10.3390/cells11193147. Cells. 2022. PMID: 36231109 Free PMC article. Review.
-
Uncovering the Expression Pattern of the Costimulatory Receptors ICOS, 4-1BB, and OX-40 in Exhausted Peripheral and Tumor-Infiltrating Natural Killer Cells from Patients with Cervical Cancer.Int J Mol Sci. 2024 Aug 12;25(16):8775. doi: 10.3390/ijms25168775. Int J Mol Sci. 2024. PMID: 39201462 Free PMC article.
-
LAG-3, TIM-3, and TIGIT: Distinct functions in immune regulation.Immunity. 2024 Feb 13;57(2):206-222. doi: 10.1016/j.immuni.2024.01.010. Immunity. 2024. PMID: 38354701 Review.
-
Defining the Immune Checkpoint Landscape in Human Colorectal Cancer Highlights the Relevance of the TIGIT/CD155 Axis for Optimizing Immunotherapy.Cancers (Basel). 2022 Aug 31;14(17):4261. doi: 10.3390/cancers14174261. Cancers (Basel). 2022. PMID: 36077799 Free PMC article.
-
CD4+ T Cells Expressing PD-1, TIGIT and LAG-3 Contribute to HIV Persistence during ART.PLoS Pathog. 2016 Jul 14;12(7):e1005761. doi: 10.1371/journal.ppat.1005761. eCollection 2016 Jul. PLoS Pathog. 2016. PMID: 27415008 Free PMC article.
References
-
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

