Osthole inhibits proliferation of human breast cancer cells by inducing cell cycle arrest and apoptosis
- PMID: 25859268
- PMCID: PMC4389113
- DOI: 10.7555/JBR.27.20120115
Osthole inhibits proliferation of human breast cancer cells by inducing cell cycle arrest and apoptosis
Abstract
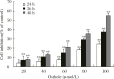
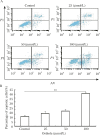
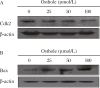
Recent studies have revealed that osthole, an active constituent isolated from the fruit of Cnidium monnieri (L.) Cusson, a traditional Chinese medicine, possesses anticancer activity. However, its effect on breast cancer cells so far has not been elucidated clearly. In the present study, we evaluated the effects of osthole on the proliferation, cell cycle and apoptosis of human breast cancer cells MDA-MB 435. We demonstrated that osthole is effective in inhibiting the proliferation of MDA-MB 435 cells, The mitochondrion-mediated apoptotic pathway was involved in apoptosis induced by osthole, as indicated by activation of caspase-9 and caspase-3 followed by PARP degradation. The mechanism underlying its effect on the induction of G1 phase arrest was due to the up-regulation of p53 and p21 and down-regulation of Cdk2 and cyclin D1 expression. Were observed taken together, these findings suggest that the anticancer efficacy of osthole is mediated via induction of cell cycle arrest and apoptosis in human breast cancer cells and osthole may be a potential chemotherapeutic agent against human breast cancer.
Keywords: apoptosis; breast cancer; cell cycle; osthole; proliferation.
Conflict of interest statement
The authors reported no conflict of interest.
Figures
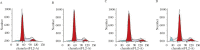


Similar articles
-
Anti-tumor effects of osthole on ovarian cancer cells in vitro.J Ethnopharmacol. 2016 Dec 4;193:368-376. doi: 10.1016/j.jep.2016.08.045. Epub 2016 Aug 24. J Ethnopharmacol. 2016. PMID: 27566206
-
Osthole Alleviates Neointimal Hyperplasia in Balloon-Induced Arterial Wall Injury by Suppressing Vascular Smooth Muscle Cell Proliferation and Downregulating Cyclin D1/CDK4 and Cyclin E1/CDK2 Expression.Front Physiol. 2021 Jan 26;11:514494. doi: 10.3389/fphys.2020.514494. eCollection 2020. Front Physiol. 2021. PMID: 33574763 Free PMC article.
-
Osthole induces G2/M cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells.Pharm Biol. 2014 May;52(5):544-50. doi: 10.3109/13880209.2013.850517. Epub 2013 Nov 15. Pharm Biol. 2014. PMID: 24236568
-
Pharmacological features of osthole.Postepy Hig Med Dosw (Online). 2017 May 15;71(0):411-421. doi: 10.5604/01.3001.0010.3824. Postepy Hig Med Dosw (Online). 2017. PMID: 28513464 Review.
-
Osthole: An up-to-date review of its anticancer potential and mechanisms of action.Front Pharmacol. 2022 Sep 7;13:945627. doi: 10.3389/fphar.2022.945627. eCollection 2022. Front Pharmacol. 2022. PMID: 36160431 Free PMC article. Review.
Cited by
-
Osthole resensitizes CD133+ hepatocellular carcinoma cells to cisplatin treatment via PTEN/AKT pathway.Aging (Albany NY). 2020 Jul 16;12(14):14406-14417. doi: 10.18632/aging.103484. Epub 2020 Jul 16. Aging (Albany NY). 2020. PMID: 32673286 Free PMC article.
-
Osthole inhibits bone metastasis of breast cancer.Oncotarget. 2017 Apr 11;8(35):58480-58493. doi: 10.18632/oncotarget.17024. eCollection 2017 Aug 29. Oncotarget. 2017. PMID: 28938572 Free PMC article.
-
Antifungal activity of osthol in vitro and enhancement in vivo through Eudragit S100 nanocarriers.Virulence. 2018 Jan 1;9(1):555-562. doi: 10.1080/21505594.2017.1356503. Virulence. 2018. PMID: 28795862 Free PMC article.
-
Osthole Ameliorates Renal Fibrosis in Mice by Suppressing Fibroblast Activation and Epithelial-Mesenchymal Transition.Front Physiol. 2018 Nov 21;9:1650. doi: 10.3389/fphys.2018.01650. eCollection 2018. Front Physiol. 2018. PMID: 30524310 Free PMC article.
-
Osthole induces apoptosis and suppresses proliferation via the PI3K/Akt pathway in intrahepatic cholangiocarcinoma.Int J Mol Med. 2017 Oct;40(4):1143-1151. doi: 10.3892/ijmm.2017.3113. Epub 2017 Aug 30. Int J Mol Med. 2017. PMID: 28902342 Free PMC article.
References
-
- Falandry C, Canney PA, Freyer G, et al. Role of combination therapy with aromatase and cyclooxygenase-2 inhibitors in patients with metastatic breast cancer. Ann Oncol. 2009;20(4):615–620. - PubMed
-
- Barnes S. Effect of genistein on in vitro and in vivo model of cancer. J nutr. 1995;125(3 Suppl):777s–783s. - PubMed
-
- Ye L, Gho WM, Chan FL, et al. Dietary administration of the licorice flavonoid isoliquiritigenin deters the growth of MCF-7 cells overespressing aromatase. Int J can. 2009;124(5):1028–1036. - PubMed
-
- Wang Y, Lee KW, Chan FL, et al. The red wine polyphenol reseveratrol displays bilevel inhibition on aromatase in breast cancer cells. Toxicol Sci. 2006;92(1):71–77. - PubMed
-
- Seo HS, Ju JH, Jang K, et al. Induction of apoptotic cell death by phytoestrogens by up-regulating the levels of phospho-p53 and p21 in normal and malignant estrogen receptor α-negative breast cells. Nutr Res. 2011;31(2):139–146. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

