The EBNA3 family of Epstein-Barr virus nuclear proteins associates with the USP46/USP12 deubiquitination complexes to regulate lymphoblastoid cell line growth
- PMID: 25855980
- PMCID: PMC4391933
- DOI: 10.1371/journal.ppat.1004822
The EBNA3 family of Epstein-Barr virus nuclear proteins associates with the USP46/USP12 deubiquitination complexes to regulate lymphoblastoid cell line growth
Abstract
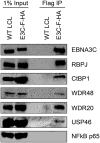
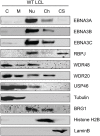
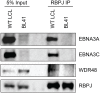
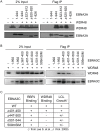
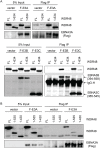
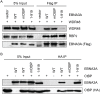
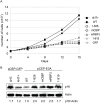
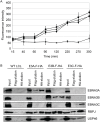
The Epstein-Barr virus (EBV) nuclear proteins EBNA3A, EBNA3B, and EBNA3C interact with the cell DNA binding protein RBPJ and regulate cell and viral genes. Repression of the CDKN2A tumor suppressor gene products p16(INK4A) and p14(ARF) by EBNA3A and EBNA3C is critical for EBV mediated transformation of resting B lymphocytes into immortalized lymphoblastoid cell lines (LCLs). To define the composition of endogenous EBNA3 protein complexes, we generated lymphoblastoid cell lines (LCLs) expressing flag-HA tagged EBNA3A, EBNA3B, or EBNA3C and used tandem affinity purification to isolate each EBNA3 complex. Our results demonstrated that each EBNA3 protein forms a distinct complex with RBPJ. Mass-spectrometry revealed that the EBNA3A and EBNA3B complexes also contained the deubquitylation complex consisting of WDR48, WDR20, and USP46 (or its paralog USP12) and that EBNA3C complexes contained WDR48. Immunoprecipitation confirmed that EBNA3A, EBNA3B, and EBNA3C association with the USP46 complex. Using chromatin immunoprecipitation, we demonstrate that WDR48 and USP46 are recruited to the p14(ARF) promoter in an EBNA3C dependent manner. Mapping studies were consistent with WDR48 being the primary mediator of EBNA3 association with the DUB complex. By ChIP assay, WDR48 was recruited to the p14(ARF) promoter in an EBNA3C dependent manner. Importantly, WDR48 associated with EBNA3A and EBNA3C domains that are critical for LCL growth, suggesting a role for USP46/USP12 in EBV induced growth transformation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



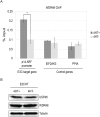
Similar articles
-
Epstein-Barr Virus Nuclear Antigen 3 (EBNA3) Proteins Regulate EBNA2 Binding to Distinct RBPJ Genomic Sites.J Virol. 2015 Dec 30;90(6):2906-19. doi: 10.1128/JVI.02737-15. J Virol. 2015. PMID: 26719268 Free PMC article.
-
Epstein-Barr Virus (EBV) Latent Protein EBNA3A Directly Targets and Silences the STK39 Gene in B Cells Infected by EBV.J Virol. 2018 Mar 14;92(7):e01918-17. doi: 10.1128/JVI.01918-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29367247 Free PMC article.
-
Core binding factor (CBF) is required for Epstein-Barr virus EBNA3 proteins to regulate target gene expression.Nucleic Acids Res. 2017 Mar 17;45(5):2368-2383. doi: 10.1093/nar/gkw1167. Nucleic Acids Res. 2017. PMID: 27903901 Free PMC article.
-
The EBNA3 Family: Two Oncoproteins and a Tumour Suppressor that Are Central to the Biology of EBV in B Cells.Curr Top Microbiol Immunol. 2015;391:61-117. doi: 10.1007/978-3-319-22834-1_3. Curr Top Microbiol Immunol. 2015. PMID: 26428372 Review.
-
The Cooperative Functions of the EBNA3 Proteins Are Central to EBV Persistence and Latency.Pathogens. 2018 Mar 17;7(1):31. doi: 10.3390/pathogens7010031. Pathogens. 2018. PMID: 29562595 Free PMC article. Review.
Cited by
-
Differential expression of miRNAs in the body wall of the sea cucumber Apostichopus japonicus under heat stress.Front Physiol. 2022 Jul 21;13:929094. doi: 10.3389/fphys.2022.929094. eCollection 2022. Front Physiol. 2022. PMID: 35936896 Free PMC article.
-
The Epstein-Barr Virus Regulome in Lymphoblastoid Cells.Cell Host Microbe. 2017 Oct 11;22(4):561-573.e4. doi: 10.1016/j.chom.2017.09.001. Cell Host Microbe. 2017. PMID: 29024646 Free PMC article.
-
Genetic Patterns Found in the Nuclear Localization Signals (NLSs) Associated with EBV-1 and EBV-2 Provide New Insights into Their Contribution to Different Cell-Type Specificities.Cancers (Basel). 2021 May 24;13(11):2569. doi: 10.3390/cancers13112569. Cancers (Basel). 2021. PMID: 34073836 Free PMC article.
-
Robust imaging and gene delivery to study human lymphoblastoid cell lines.J Hum Genet. 2018 Sep;63(9):945-955. doi: 10.1038/s10038-018-0483-2. Epub 2018 Jun 20. J Hum Genet. 2018. PMID: 29925960
-
The novel anti-androgen candidate galeterone targets deubiquitinating enzymes, USP12 and USP46, to control prostate cancer growth and survival.Oncotarget. 2018 May 18;9(38):24992-25007. doi: 10.18632/oncotarget.25167. eCollection 2018 May 18. Oncotarget. 2018. PMID: 29861848 Free PMC article.
References
-
- Rickinson AB, Kieff E (2007) Epstein-Barr Virus. Philadelphia: Lippincott Williams and Wilkins; 2655–2700 p.
-
- Henle W, Diehl V, Kohn G, Zur Hausen H, Henle G (1967) Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science 157: 1064–1065. - PubMed
-
- Pope JH, Horne MK, Scott W (1968) Transformation of foetal human keukocytes in vitro by filtrates of a human leukaemic cell line containing herpes-like virus. International journal of cancer 3: 857–866. - PubMed
-
- Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, et al. (1998) Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood 92: 1549–1555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

