Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells
- PMID: 25837513
- PMCID: PMC4549796
- DOI: 10.1126/science.aaa3828
Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells
Abstract
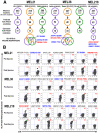
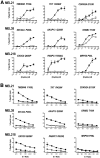
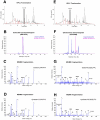
T cell immunity directed against tumor-encoded amino acid substitutions occurs in some melanoma patients. This implicates missense mutations as a source of patient-specific neoantigens. However, a systematic evaluation of these putative neoantigens as targets of antitumor immunity is lacking. Moreover, it remains unknown whether vaccination can augment such responses. We found that a dendritic cell vaccine led to an increase in naturally occurring neoantigen-specific immunity and revealed previously undetected human leukocyte antigen (HLA) class I-restricted neoantigens in patients with advanced melanoma. The presentation of neoantigens by HLA-A*02:01 in human melanoma was confirmed by mass spectrometry. Vaccination promoted a diverse neoantigen-specific T cell receptor (TCR) repertoire in terms of both TCR-β usage and clonal composition. Our results demonstrate that vaccination directed at tumor-encoded amino acid substitutions broadens the antigenic breadth and clonal diversity of antitumor immunity.
Trial registration: ClinicalTrials.gov NCT00683670.
Copyright © 2015, American Association for the Advancement of Science.
Figures

Comment in
-
Cancer immunotherapy. Neo approaches to cancer vaccines.Science. 2015 May 15;348(6236):760-1. doi: 10.1126/science.aab3465. Science. 2015. PMID: 25977539 No abstract available.
-
Clinical genetics. Sequencing for tailored melanoma immunotherapy.Nat Rev Genet. 2015 May;16(5):259. doi: 10.1038/nrg3945. Nat Rev Genet. 2015. PMID: 26065036 No abstract available.
Similar articles
-
Immunological ignorance is an enabling feature of the oligo-clonal T cell response to melanoma neoantigens.Proc Natl Acad Sci U S A. 2019 Nov 19;116(47):23662-23670. doi: 10.1073/pnas.1906026116. Epub 2019 Nov 4. Proc Natl Acad Sci U S A. 2019. PMID: 31685621 Free PMC article.
-
Cancer immunotherapy. Neo approaches to cancer vaccines.Science. 2015 May 15;348(6236):760-1. doi: 10.1126/science.aab3465. Science. 2015. PMID: 25977539 No abstract available.
-
Clinical genetics. Sequencing for tailored melanoma immunotherapy.Nat Rev Genet. 2015 May;16(5):259. doi: 10.1038/nrg3945. Nat Rev Genet. 2015. PMID: 26065036 No abstract available.
-
Review: dendritic cell immunotherapy for melanoma.Cancer Biother Radiopharm. 1999 Feb;14(1):11-22. doi: 10.1089/cbr.1999.14.11. Cancer Biother Radiopharm. 1999. PMID: 10850282 Review.
-
T-cell-directed cancer vaccines: the melanoma model.Expert Opin Biol Ther. 2001 Mar;1(2):277-90. doi: 10.1517/14712598.1.2.277. Expert Opin Biol Ther. 2001. PMID: 11727535 Review.
Cited by
-
Neoantigen vaccine platforms in clinical development: understanding the future of personalized immunotherapy.Expert Opin Investig Drugs. 2021 May;30(5):529-541. doi: 10.1080/13543784.2021.1896702. Epub 2021 Mar 31. Expert Opin Investig Drugs. 2021. PMID: 33641576 Free PMC article. Review.
-
Antitumour dendritic cell vaccination in a priming and boosting approach.Nat Rev Drug Discov. 2020 Sep;19(9):635-652. doi: 10.1038/s41573-020-0074-8. Epub 2020 Aug 6. Nat Rev Drug Discov. 2020. PMID: 32764681 Review.
-
Challenges and Opportunities for Pancreatic Cancer Immunotherapy.Cancer Cell. 2020 Dec 14;38(6):788-802. doi: 10.1016/j.ccell.2020.08.004. Epub 2020 Sep 17. Cancer Cell. 2020. PMID: 32946773 Free PMC article. Review.
-
Short Peptides as Powerful Arsenal for Smart Fighting Cancer.Cancers (Basel). 2024 Sep 24;16(19):3254. doi: 10.3390/cancers16193254. Cancers (Basel). 2024. PMID: 39409876 Free PMC article. Review.
-
A poly-neoantigen DNA vaccine synergizes with PD-1 blockade to induce T cell-mediated tumor control.Oncoimmunology. 2019 Sep 2;8(11):1652539. doi: 10.1080/2162402X.2019.1652539. eCollection 2019. Oncoimmunology. 2019. PMID: 31646082 Free PMC article.
References
-
- Wolfel T, et al. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 1995;269:1281. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

