CD133+ ovarian cancer stem-like cells promote non-stem cancer cell metastasis via CCL5 induced epithelial-mesenchymal transition
- PMID: 25788271
- PMCID: PMC4467406
- DOI: 10.18632/oncotarget.3462
CD133+ ovarian cancer stem-like cells promote non-stem cancer cell metastasis via CCL5 induced epithelial-mesenchymal transition
Abstract
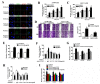
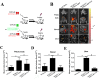
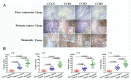
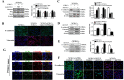
Cancer stem cells (CSCs, also called cancer stem-like cells, CSLCs) can function as "seed cells" for tumor recurrence and metastasis. Here, we report that, in the presence of CD133+ ovarian CSLCs, CD133- non-CSLCs can undergo an epithelial-mesenchymal transition (EMT)-like process and display enhanced metastatic capacity in vitro and in vivo. Highly elevated expression of chemokine (C-C motif) ligand 5 (CCL5) and its receptors chemokine (C-C motif) receptor (CCR) 1/3/5 are observed in clinical and murine metastatic tumor tissues from epithelial ovarian carcinomas. Mechanistically, paracrine CCL5 from ovarian CSLCs activates the NF-κB signaling pathway in ovarian non-CSLCs via binding CCR1/3/5, thereby inducing EMT and tumor invasion. Taken together, our results redefine the metastatic potential of non-stem cancer cells and provide evidence that targeting the CCL5:CCR1/3/5-NF-κB pathway could be an effective strategy to prevent ovarian cancer metastasis.
Keywords: NF-κB; cancer stem like cells; chemokine (C-C motif) ligand 5; epithelial-mesenchymal transition; non-cancer stem like cells.
Conflict of interest statement
All authors declare no potential conflicts of interest.
Figures


Similar articles
-
Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-κB-mediated MMP-9 upregulation.Stem Cells. 2012 Oct;30(10):2309-19. doi: 10.1002/stem.1194. Stem Cells. 2012. PMID: 22887854
-
Ovarian cancer stem-like cells differentiate into endothelial cells and participate in tumor angiogenesis through autocrine CCL5 signaling.Cancer Lett. 2016 Jun 28;376(1):137-47. doi: 10.1016/j.canlet.2016.03.034. Epub 2016 Mar 23. Cancer Lett. 2016. PMID: 27033454
-
Interleukin-17 produced by tumor microenvironment promotes self-renewal of CD133+ cancer stem-like cells in ovarian cancer.Oncogene. 2015 Jan 8;34(2):165-76. doi: 10.1038/onc.2013.537. Epub 2013 Dec 23. Oncogene. 2015. PMID: 24362529
-
Mechanism for the Decision of Ovarian Surface Epithelial Stem Cells to Undergo Neo-Oogenesis or Ovarian Tumorigenesis.Cell Physiol Biochem. 2018;50(1):214-232. doi: 10.1159/000494001. Epub 2018 Oct 18. Cell Physiol Biochem. 2018. PMID: 30336465 Review.
-
Cancer stem cells, epithelial-mesenchymal transition, and drug resistance in high-grade ovarian serous carcinoma.Hum Pathol. 2013 Nov;44(11):2373-84. doi: 10.1016/j.humpath.2013.05.001. Epub 2013 Jul 11. Hum Pathol. 2013. PMID: 23850493 Free PMC article. Review.
Cited by
-
Cancer Cells Exploit Notch Signaling to Redefine a Supportive Cytokine Milieu.Front Immunol. 2018 Aug 14;9:1823. doi: 10.3389/fimmu.2018.01823. eCollection 2018. Front Immunol. 2018. PMID: 30154786 Free PMC article. Review.
-
Clinical effects of TP-based hyperthermic intraperitoneal chemotherapy (HIPEC) on CD133 and HE4 expression in advanced epithelial ovarian cancer.Pak J Med Sci. 2022 Jul-Aug;38(6):1508-1513. doi: 10.12669/pjms.38.6.5287. Pak J Med Sci. 2022. PMID: 35991244 Free PMC article.
-
Targeting the Epithelial-to-Mesenchymal Transition: The Case for Differentiation-Based Therapy.Cold Spring Harb Symp Quant Biol. 2016;81:11-19. doi: 10.1101/sqb.2016.81.030957. Epub 2017 Jan 5. Cold Spring Harb Symp Quant Biol. 2016. PMID: 28057845 Free PMC article. Review.
-
Chemotherapy induces adaptive drug resistance and metastatic potentials via phenotypic CXCR4-expressing cell state transition in ovarian cancer.PLoS One. 2017 Feb 14;12(2):e0171044. doi: 10.1371/journal.pone.0171044. eCollection 2017. PLoS One. 2017. PMID: 28196146 Free PMC article.
-
Epithelial-to-mesenchymal transition in cancer: complexity and opportunities.Front Med. 2018 Aug;12(4):361-373. doi: 10.1007/s11684-018-0656-6. Epub 2018 Jul 24. Front Med. 2018. PMID: 30043221 Free PMC article. Review.
References
-
- Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

