Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells
- PMID: 25787765
- PMCID: PMC4425193
- DOI: 10.1126/scitranslmed.3010302
Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells
Abstract
The skin of an adult human contains about 20 billion memory T cells. Epithelial barrier tissues are infiltrated by a combination of resident and recirculating T cells in mice, but the relative proportions and functional activities of resident versus recirculating T cells have not been evaluated in human skin. We discriminated resident from recirculating T cells in human-engrafted mice and lymphoma patients using alemtuzumab, a medication that depletes recirculating T cells from skin, and then analyzed these T cell populations in healthy human skin. All nonrecirculating resident memory T cells (TRM) expressed CD69, but most were CD4(+), CD103(-), and located in the dermis, in contrast to studies in mice. Both CD4(+) and CD8(+) CD103(+) TRM were enriched in the epidermis, had potent effector functions, and had a limited proliferative capacity compared to CD103(-) TRM. TRM of both types had more potent effector functions than recirculating T cells. We observed two distinct populations of recirculating T cells, CCR7(+)/L-selectin(+) central memory T cells (TCM) and CCR7(+)/L-selectin(-) T cells, which we term migratory memory T cells (TMM). Circulating skin-tropic TMM were intermediate in cytokine production between TCM and effector memory T cells. In patients with cutaneous T cell lymphoma, malignant TCM and TMM induced distinct inflammatory skin lesions, and TMM were depleted more slowly from skin after alemtuzumab, suggesting that TMM may recirculate more slowly. In summary, human skin is protected by four functionally distinct populations of T cells, two resident and two recirculating, with differing territories of migration and distinct functional activities.
Copyright © 2015, American Association for the Advancement of Science.
Conflict of interest statement
Figures

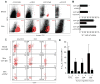

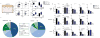
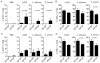
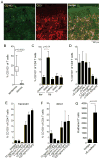

Similar articles
-
Functional Heterogeneity of CD4+ Tumor-Infiltrating Lymphocytes With a Resident Memory Phenotype in NSCLC.Front Immunol. 2018 Nov 16;9:2654. doi: 10.3389/fimmu.2018.02654. eCollection 2018. Front Immunol. 2018. PMID: 30505306 Free PMC article.
-
Human intrahepatic CD69 + CD8+ T cells have a tissue resident memory T cell phenotype with reduced cytolytic capacity.Sci Rep. 2017 Jul 21;7(1):6172. doi: 10.1038/s41598-017-06352-3. Sci Rep. 2017. PMID: 28733665 Free PMC article.
-
Distinct recirculation potential of CD69+CD103- and CD103+ thymic memory CD8+ T cells.Immunol Cell Biol. 2016 Nov;94(10):975-980. doi: 10.1038/icb.2016.60. Epub 2016 Jun 22. Immunol Cell Biol. 2016. PMID: 27328704
-
Tissue-resident memory T cells in the skin.Inflamm Res. 2020 Mar;69(3):245-254. doi: 10.1007/s00011-020-01320-6. Epub 2020 Jan 27. Inflamm Res. 2020. PMID: 31989191 Review.
-
The Emerging Role of CD8+ Tissue Resident Memory T (TRM) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin.Front Immunol. 2018 Aug 15;9:1904. doi: 10.3389/fimmu.2018.01904. eCollection 2018. Front Immunol. 2018. PMID: 30158938 Free PMC article. Review.
Cited by
-
The influence of skin microorganisms on cutaneous immunity.Nat Rev Immunol. 2016 May 27;16(6):353-66. doi: 10.1038/nri.2016.48. Nat Rev Immunol. 2016. PMID: 27231051 Review.
-
Dihydroartemisinin ameliorates psoriatic skin inflammation and its relapse by diminishing CD8+ T-cell memory in wild-type and humanized mice.Theranostics. 2020 Aug 21;10(23):10466-10482. doi: 10.7150/thno.45211. eCollection 2020. Theranostics. 2020. PMID: 32929360 Free PMC article.
-
Recent advances in understanding psoriasis.F1000Res. 2016 Apr 28;5:F1000 Faculty Rev-770. doi: 10.12688/f1000research.7927.1. eCollection 2016. F1000Res. 2016. PMID: 27158469 Free PMC article. Review.
-
Divergence of Tissue-Memory T Cells: Distribution and Function-Based Classification.Cold Spring Harb Perspect Biol. 2020 Oct 1;12(10):a037762. doi: 10.1101/cshperspect.a037762. Cold Spring Harb Perspect Biol. 2020. PMID: 32816841 Free PMC article. Review.
-
Functional Heterogeneity of CD4+ Tumor-Infiltrating Lymphocytes With a Resident Memory Phenotype in NSCLC.Front Immunol. 2018 Nov 16;9:2654. doi: 10.3389/fimmu.2018.02654. eCollection 2018. Front Immunol. 2018. PMID: 30505306 Free PMC article.
References
-
- Carbone FR, Mackay LK, Heath WR, Gebhardt T. Distinct resident and recirculating memory T cell subsets in non-lymphoid tissues. Current opinion in immunology. 2013 Jun;25:329. - PubMed
-
- Siders WM, et al. Involvement of neutrophils and natural killer cells in the anti-tumor activity of alemtuzumab in xenograft tumor models. Leukemia & lymphoma. 2010 Jul;51:1293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

