SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx
- PMID: 25730848
- PMCID: PMC4352781
- DOI: 10.1073/pnas.1414055112
SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx
Abstract
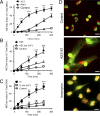
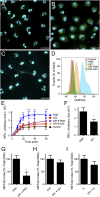
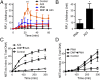
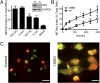
Neutrophils cast neutrophil extracellular traps (NETs) to defend the host against invading pathogens. Although effective against microbial pathogens, a growing body of literature now suggests that NETs have negative impacts on many inflammatory and autoimmune diseases. Identifying mechanisms that regulate the process termed "NETosis" is important for treating these diseases. Although two major types of NETosis have been described to date, mechanisms regulating these forms of cell death are not clearly established. NADPH oxidase 2 (NOX2) generates large amounts of reactive oxygen species (ROS), which is essential for NOX-dependent NETosis. However, major regulators of NOX-independent NETosis are largely unknown. Here we show that calcium activated NOX-independent NETosis is fast and mediated by a calcium-activated small conductance potassium (SK) channel member SK3 and mitochondrial ROS. Although mitochondrial ROS is needed for NOX-independent NETosis, it is not important for NOX-dependent NETosis. We further demonstrate that the activation of the calcium-activated potassium channel is sufficient to induce NOX-independent NETosis. Unlike NOX-dependent NETosis, NOX-independent NETosis is accompanied by a substantially lower level of activation of ERK and moderate level of activation of Akt, whereas the activation of p38 is similar in both pathways. ERK activation is essential for the NOX-dependent pathway, whereas its activation is not essential for the NOX-independent pathway. Despite the differential activation, both NOX-dependent and -independent NETosis require Akt activity. Collectively, this study highlights key differences in these two major NETosis pathways and provides an insight into previously unknown mechanisms for NOX-independent NETosis.
Keywords: NADPH oxidase; NETosis; SK channels; neutrophil extracellular traps; neutrophils.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Histone Acetylation Promotes Neutrophil Extracellular Trap Formation.Biomolecules. 2019 Jan 18;9(1):32. doi: 10.3390/biom9010032. Biomolecules. 2019. PMID: 30669408 Free PMC article.
-
Entamoeba histolytica Trophozoites Induce a Rapid Non-classical NETosis Mechanism Independent of NOX2-Derived Reactive Oxygen Species and PAD4 Activity.Front Cell Infect Microbiol. 2018 Jun 5;8:184. doi: 10.3389/fcimb.2018.00184. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29922599 Free PMC article.
-
Mitochondrial permeability transition pore is involved in oxidative burst and NETosis of human neutrophils.Biochim Biophys Acta Mol Basis Dis. 2020 May 1;1866(5):165664. doi: 10.1016/j.bbadis.2020.165664. Epub 2020 Jan 8. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 31926265
-
How Do ROS Induce NETosis? Oxidative DNA Damage, DNA Repair, and Chromatin Decondensation.Biomolecules. 2024 Oct 16;14(10):1307. doi: 10.3390/biom14101307. Biomolecules. 2024. PMID: 39456240 Free PMC article. Review.
-
NOX2-dependent regulation of inflammation.Clin Sci (Lond). 2016 Apr 1;130(7):479-90. doi: 10.1042/CS20150660. Clin Sci (Lond). 2016. PMID: 26888560 Free PMC article. Review.
Cited by
-
Recent Insights into Neutrophil Extracellular Traps in Cardiovascular Diseases.J Clin Med. 2022 Nov 10;11(22):6662. doi: 10.3390/jcm11226662. J Clin Med. 2022. PMID: 36431139 Free PMC article. Review.
-
The emerging role of neutrophil extracellular traps in ulcerative colitis.Front Immunol. 2024 Aug 7;15:1425251. doi: 10.3389/fimmu.2024.1425251. eCollection 2024. Front Immunol. 2024. PMID: 39170617 Free PMC article. Review.
-
NETosing Neutrophils Activate Complement Both on Their Own NETs and Bacteria via Alternative and Non-alternative Pathways.Front Immunol. 2016 Apr 14;7:137. doi: 10.3389/fimmu.2016.00137. eCollection 2016. Front Immunol. 2016. PMID: 27148258 Free PMC article.
-
Neutrophils in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus: Same Foe Different M.O.Front Immunol. 2021 Mar 4;12:649693. doi: 10.3389/fimmu.2021.649693. eCollection 2021. Front Immunol. 2021. PMID: 33746988 Free PMC article. Review.
-
Helminth derived factors inhibit neutrophil extracellular trap formation and inflammation in bacterial peritonitis.Sci Rep. 2021 Jun 16;11(1):12718. doi: 10.1038/s41598-021-92001-9. Sci Rep. 2021. PMID: 34135384 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

