Bisphenol-A induces expression of HOXC6, an estrogen-regulated homeobox-containing gene associated with breast cancer
- PMID: 25725483
- PMCID: PMC4437882
- DOI: 10.1016/j.bbagrm.2015.02.003
Bisphenol-A induces expression of HOXC6, an estrogen-regulated homeobox-containing gene associated with breast cancer
Abstract
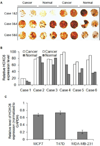
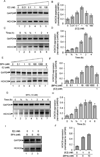
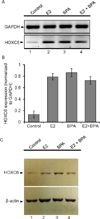
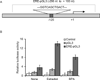
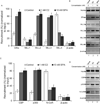
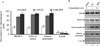
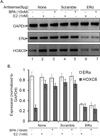
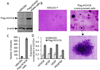
HOXC6 is a homeobox-containing gene associated with mammary gland development and is overexpressed in variety of cancers including breast and prostate cancers. Here, we have examined the expression of HOXC6 in breast cancer tissue, investigated its transcriptional regulation via estradiol (E2) and bisphenol-A (BPA, an estrogenic endocrine disruptor) in vitro and in vivo. We observed that HOXC6 is differentially over-expressed in breast cancer tissue. E2 induces HOXC6 expression in cultured breast cancer cells and in mammary glands of Sprague Dawley rats. HOXC6 expression is also induced upon exposure to BPA both in vitro and in vivo. Estrogen-receptor-alpha (ERα) and ER-coregulators such as MLL-histone methylases are bound to the HOXC6 promoter upon exposure to E2 or BPA and that resulted in increased histone H3K4-trimethylation, histone acetylation, and recruitment of RNA polymerase II at the HOXC6 promoter. HOXC6 overexpression induces expression of tumor growth factors and facilitates growth 3D-colony formation, indicating its potential roles in tumor growth. Our studies demonstrate that HOXC6, which is a critical player in mammary gland development, is upregulated in multiple cases of breast cancer, and is transcriptionally regulated by E2 and BPA, in vitro and in vivo.
Keywords: Bisphenol A; Endocrine disruption; Epigenetics; Estrogen-receptors; HOXC6 expression; Mixed lineage leukemia (MLL).
Published by Elsevier B.V.
Figures
Similar articles
-
Endocrine disrupting chemical, bisphenol-A, induces breast cancer associated gene HOXB9 expression in vitro and in vivo.Gene. 2016 Sep 30;590(2):234-43. doi: 10.1016/j.gene.2016.05.009. Epub 2016 May 13. Gene. 2016. PMID: 27182052 Free PMC article.
-
HOXC6 Is transcriptionally regulated via coordination of MLL histone methylase and estrogen receptor in an estrogen environment.J Mol Biol. 2011 Aug 12;411(2):334-49. doi: 10.1016/j.jmb.2011.05.050. Epub 2011 Jun 12. J Mol Biol. 2011. PMID: 21683083 Free PMC article.
-
Bisphenol-A and diethylstilbestrol exposure induces the expression of breast cancer associated long noncoding RNA HOTAIR in vitro and in vivo.J Steroid Biochem Mol Biol. 2014 May;141:160-70. doi: 10.1016/j.jsbmb.2014.02.002. Epub 2014 Feb 14. J Steroid Biochem Mol Biol. 2014. PMID: 24533973 Free PMC article.
-
Stop eating plastic, molecular signaling of bisphenol A in breast cancer.Environ Sci Pollut Res Int. 2018 Aug;25(24):23624-23630. doi: 10.1007/s11356-018-2540-y. Epub 2018 Jun 29. Environ Sci Pollut Res Int. 2018. PMID: 29959737 Review.
-
The promiscuous estrogen receptor: Evolution of physiological estrogens and response to phytochemicals and endocrine disruptors.J Steroid Biochem Mol Biol. 2018 Nov;184:29-37. doi: 10.1016/j.jsbmb.2018.07.001. Epub 2018 Jul 20. J Steroid Biochem Mol Biol. 2018. PMID: 30009950 Review.
Cited by
-
Research on radiotherapy related genes and prognostic target identification of rectal cancer based on multi-omics.J Transl Med. 2023 Nov 27;21(1):856. doi: 10.1186/s12967-023-04753-9. J Transl Med. 2023. PMID: 38012642 Free PMC article.
-
The State of Research and Weight of Evidence on the Epigenetic Effects of Bisphenol A.Int J Mol Sci. 2023 Apr 27;24(9):7951. doi: 10.3390/ijms24097951. Int J Mol Sci. 2023. PMID: 37175656 Free PMC article.
-
Current Knowledge on Endocrine Disrupting Chemicals (EDCs) from Animal Biology to Humans, from Pregnancy to Adulthood: Highlights from a National Italian Meeting.Int J Mol Sci. 2018 Jun 2;19(6):1647. doi: 10.3390/ijms19061647. Int J Mol Sci. 2018. PMID: 29865233 Free PMC article. Review.
-
Epigenetic Alteration Shaped by the Environmental Chemical Bisphenol A.Front Genet. 2021 Jan 11;11:618966. doi: 10.3389/fgene.2020.618966. eCollection 2020. Front Genet. 2021. PMID: 33505438 Free PMC article. Review.
-
LncRNA HOTAIR: A master regulator of chromatin dynamics and cancer.Biochim Biophys Acta. 2015 Aug;1856(1):151-64. doi: 10.1016/j.bbcan.2015.07.001. Epub 2015 Jul 21. Biochim Biophys Acta. 2015. PMID: 26208723 Free PMC article. Review.
References
-
- Yu BD, Hess JL, Horning SE, Brown GA, Korsmeyer SJ. Altered Hox expression and segmental identity in Mll-mutant mice. Nature. 1995;378:505–508. - PubMed
-
- Alexander T, Nolte C, Krumlauf R. Hox genes and segmentation of the hindbrain and axial skeleton. Annu Rev Cell Dev Biol. 2009;25:431–456. - PubMed
-
- Takahashi Y, Hamada J-i, Murakawa K, Takada M, Tada M, Nogami I, Hayashi N, Nakamori S, Monden M, Miyamoto M, Katoh H, Moriuchi T. Expression profiles of 39 HOX genes in normal human adult organs and anaplastic thyroid cancer cell lines by quantitative real-time RT-PCR system. Experimental Cell Research. 2004;293:144–153. - PubMed
-
- Mallo M, Alonso CR. The regulation of Hox gene expression during animal development. Development. 2013;140:3951–3963. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

