Immature myeloid cells directly contribute to skin tumor development by recruiting IL-17-producing CD4+ T cells
- PMID: 25667306
- PMCID: PMC4354367
- DOI: 10.1084/jem.20140835
Immature myeloid cells directly contribute to skin tumor development by recruiting IL-17-producing CD4+ T cells
Abstract
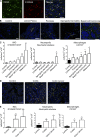
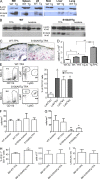
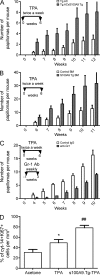
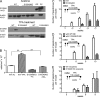
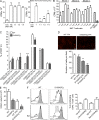
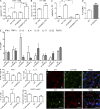
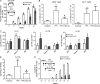
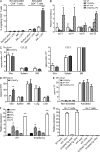
Evidence links chronic inflammation with cancer, but cellular mechanisms involved in this process remain unclear. We have demonstrated that in humans, inflammatory conditions that predispose to development of skin and colon tumors are associated with accumulation in tissues of CD33+S100A9+ cells, the phenotype typical for myeloid-derived suppressor cells in cancer or immature myeloid cells (IMCs) in tumor-free hosts. To identify the direct role of these cells in tumor development, we used S100A9 transgenic mice to create the conditions for topical accumulation of these cells in the skin in the absence of infection or tissue damage. These mice demonstrated accumulation of granulocytic IMCs in the skin upon topical application of 12-O-tetradecanoylphorbol-13-acetate (TPA), resulting in a dramatic increase in the formation of papillomas during epidermal carcinogenesis. The effect of IMCs on tumorigenesis was not associated with immune suppression, but with CCL4 (chemokine [C-C motif] ligand 4)-mediated recruitment of IL-17-producing CD4+ T cells. This chemokine was released by activated IMCs. Elimination of CD4+ T cells or blockade of CCL4 or IL-17 abrogated the increase in tumor formation caused by myeloid cells. Thus, this study implicates accumulation of IMCs as an initial step in facilitation of tumor formation, followed by the recruitment of CD4+ T cells.
© 2015 Ortiz et al.
Figures
Similar articles
-
S100A9 has a protective role in inflammation-induced skin carcinogenesis.Int J Cancer. 2014 Aug 15;135(4):798-808. doi: 10.1002/ijc.28725. Epub 2014 Jan 28. Int J Cancer. 2014. PMID: 24436096
-
Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth.J Immunol. 2012 Dec 15;189(12):5602-11. doi: 10.4049/jimmunol.1201018. Epub 2012 Nov 14. J Immunol. 2012. PMID: 23152559
-
Myeloid TGF-β signaling contributes to colitis-associated tumorigenesis in mice.Carcinogenesis. 2013 Sep;34(9):2099-108. doi: 10.1093/carcin/bgt172. Epub 2013 May 21. Carcinogenesis. 2013. PMID: 23695722
-
All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination.Cancer Res. 2003 Aug 1;63(15):4441-9. Cancer Res. 2003. PMID: 12907617
-
Reciprocal relationship between myeloid-derived suppressor cells and T cells.J Immunol. 2013 Jul 1;191(1):17-23. doi: 10.4049/jimmunol.1300654. J Immunol. 2013. PMID: 23794702 Free PMC article. Review.
Cited by
-
Myeloid-derived suppressor cells: The green light for myeloma immune escape.Blood Rev. 2016 Sep;30(5):341-8. doi: 10.1016/j.blre.2016.04.002. Epub 2016 Apr 12. Blood Rev. 2016. PMID: 27132116 Free PMC article. Review.
-
The IL-17 family in diseases: from bench to bedside.Signal Transduct Target Ther. 2023 Oct 11;8(1):402. doi: 10.1038/s41392-023-01620-3. Signal Transduct Target Ther. 2023. PMID: 37816755 Free PMC article. Review.
-
The downregulation of type I IFN signaling in G-MDSCs under tumor conditions promotes their development towards an immunosuppressive phenotype.Cell Death Dis. 2022 Jan 10;13(1):36. doi: 10.1038/s41419-021-04487-w. Cell Death Dis. 2022. PMID: 35013108 Free PMC article.
-
Galectin-7 reprograms skin carcinogenesis by fostering innate immune evasive programs.Cell Death Differ. 2023 Apr;30(4):906-921. doi: 10.1038/s41418-022-01108-7. Epub 2023 Jan 24. Cell Death Differ. 2023. PMID: 36693903 Free PMC article.
-
The dysfunction of BP180/collagen XVII in keratinocytes promotes melanoma progression.Oncogene. 2019 Dec;38(50):7491-7503. doi: 10.1038/s41388-019-0961-9. Epub 2019 Aug 21. Oncogene. 2019. PMID: 31435021 Free PMC article.
References
-
- Brandau S., Trellakis S., Bruderek K., Schmaltz D., Steller G., Elian M., Suttmann H., Schenck M., Welling J., Zabel P., and Lang S.. 2011. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J. Leukoc. Biol. 89:311–317 10.1189/jlb.0310162 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

