Honokiol confers immunogenicity by dictating calreticulin exposure, activating ER stress and inhibiting epithelial-to-mesenchymal transition
- PMID: 25619450
- PMCID: PMC5528772
- DOI: 10.1016/j.molonc.2014.12.009
Honokiol confers immunogenicity by dictating calreticulin exposure, activating ER stress and inhibiting epithelial-to-mesenchymal transition
Abstract
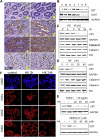
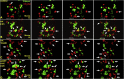
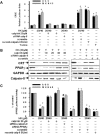
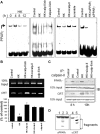
Peritoneal dissemination is a major clinical obstacle in gastrointestinal cancer therapy, and it accounts for the majority of cancer-related mortality. Calreticulin (CRT) is over-expressed in gastric tumors and has been linked to poor prognosis. In this study, immunohistochemistry studies revealed that the up-regulation of CRT was associated with lymph node and distant metastasis in patients with gastric cancer specimens. CRT was significantly down-regulated in highly metastatic gastric cancer cell lines and metastatic animal by Honokiol-treated. Small RNA interference blocking CRT by siRNA-CRT was translocated to the cells in the early immunogenic response to Honokiol. Honokiol activated endoplasmic reticulum (ER) stress and down-regulated peroxisome proliferator-activated receptor-γ (PPARγ) activity resulting in PPARγ and CRT degradation through calpain-II activity, which could be reversed by siRNA-calpain-II. The Calpain-II/PPARγ/CRT axis and interaction evoked by Honokiol could be blocked by gene silencing or pharmacological agents. Both transforming growth factor (TGF)-β1 and N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) induced cell migration, invasion and reciprocal down-regulation of epithelial marker E-cadherin, which could be abrogated by siRNA-CRT. Moreover, Honokiol significantly suppressed MNNG-induced gastrointestinal tumor growth and over-expression of CRT in mice. Knockdown CRT in gastric cancer cells was found to effectively reduce growth ability and metastasis in vivo. The present study provides insight into the specific biological behavior of CRT in epithelial-to-mesenchymal transition (EMT) and metastasis. Taken together, our results suggest that the therapeutic inhibition of CRT by Honokiol suppresses both gastric tumor growth and peritoneal dissemination by dictating early translocation of CRT in immunogenic cell death, activating ER stress, and blocking EMT.
Keywords: Calreticulin; Carcinogenesis; Endoplasmic reticulum stress; Epithelial-to-mesenchymal transition; Immunogenic cell death.
Copyright © 2015 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Targeting histone deacetylase-3 blocked epithelial-mesenchymal plasticity and metastatic dissemination in gastric cancer.Cell Biol Toxicol. 2023 Oct;39(5):1873-1896. doi: 10.1007/s10565-021-09673-2. Epub 2022 Jan 1. Cell Biol Toxicol. 2023. PMID: 34973135 Free PMC article.
-
Honokiol thwarts gastric tumor growth and peritoneal dissemination by inhibiting Tpl2 in an orthotopic model.Carcinogenesis. 2013 Nov;34(11):2568-79. doi: 10.1093/carcin/bgt243. Epub 2013 Jul 4. Carcinogenesis. 2013. PMID: 23828905
-
Exploiting Honokiol-induced ER stress CHOP activation inhibits the growth and metastasis of melanoma by suppressing the MITF and β-catenin pathways.Cancer Lett. 2019 Feb 1;442:113-125. doi: 10.1016/j.canlet.2018.10.026. Epub 2018 Nov 1. Cancer Lett. 2019. PMID: 30391358
-
Calreticulin as a marker and therapeutic target for cancer.Clin Exp Med. 2023 Sep;23(5):1393-1404. doi: 10.1007/s10238-022-00937-7. Epub 2022 Nov 6. Clin Exp Med. 2023. PMID: 36335525 Review.
-
Use of honokiol in lung cancer therapy: a mini review of its pharmacological mechanism.J Asian Nat Prod Res. 2023 Nov;25(11):1029-1037. doi: 10.1080/10286020.2023.2193695. Epub 2023 Apr 3. J Asian Nat Prod Res. 2023. PMID: 37010929 Review.
Cited by
-
Honokiol: A Review of Its Anticancer Potential and Mechanisms.Cancers (Basel). 2019 Dec 22;12(1):48. doi: 10.3390/cancers12010048. Cancers (Basel). 2019. PMID: 31877856 Free PMC article. Review.
-
Targeting histone deacetylase-3 blocked epithelial-mesenchymal plasticity and metastatic dissemination in gastric cancer.Cell Biol Toxicol. 2023 Oct;39(5):1873-1896. doi: 10.1007/s10565-021-09673-2. Epub 2022 Jan 1. Cell Biol Toxicol. 2023. PMID: 34973135 Free PMC article.
-
Cardiovascular Adiponectin Resistance: The Critical Role of Adiponectin Receptor Modification.Trends Endocrinol Metab. 2017 Jul;28(7):519-530. doi: 10.1016/j.tem.2017.03.004. Epub 2017 May 1. Trends Endocrinol Metab. 2017. PMID: 28473178 Free PMC article. Review.
-
Targeting LncRNA-MALAT1 suppresses the progression of osteosarcoma by altering the expression and localization of β-catenin.J Cancer. 2018 Jan 1;9(1):71-80. doi: 10.7150/jca.22113. eCollection 2018. J Cancer. 2018. PMID: 29290771 Free PMC article.
-
EVI‑1 acts as an oncogene and positively regulates calreticulin in breast cancer.Mol Med Rep. 2019 Mar;19(3):1645-1653. doi: 10.3892/mmr.2018.9796. Epub 2018 Dec 24. Mol Med Rep. 2019. Retraction in: Mol Med Rep. 2023 Dec;28(6):225. doi: 10.3892/mmr.2023.13112 PMID: 30592274 Free PMC article. Retracted.
References
-
- Chao, M.P. , Majeti, R. , Weissman, I.L. , 2012. Programmed cell removal: a new obstacle in the road to developing cancer. Nat. Rev. Cancer 12, 58–67. - PubMed
-
- Chen, C.N. , Chang, C.C. , Su, T.E. , Hsu, W.M. , Jeng, Y.M. , Ho, M.C. , Hsieh, F.J. , Lee, P.H. , Kuo, M.L. , Lee, H. , Chang, K.J. , 2009. Identification of calreticulin as a prognosis marker and angiogenic regulator in human gastric cancer. Ann. Surg. Oncol. 16, 524–533. - PubMed
-
- Chen, C.N. , Lin, J.J. , Lee, H. , Cheng, Y.M. , Chang, K.J. , Hsieh, F.J. , Lai, H.S. , Chang, C.C. , Lee, P.H. , 2009. Association between color Doppler vascularity index, angiogenesis-related molecules, and clinical outcomes in gastric cancer. J. Surg. Oncol. 99, 402–408. - PubMed
-
- De, C.B. , Berx, G. , 2013. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 13, 97–110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

