Distribution and frequencies of post-transcriptional modifications in tRNAs
- PMID: 25611331
- PMCID: PMC4615829
- DOI: 10.4161/15476286.2014.992273
Distribution and frequencies of post-transcriptional modifications in tRNAs
Abstract
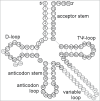
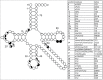
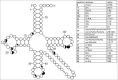
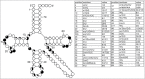
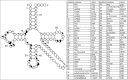
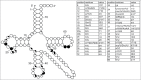
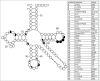
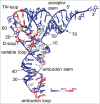
Functional tRNA molecules always contain a wide variety of post-transcriptionally modified nucleosides. These modifications stabilize tRNA structure, allow for proper interaction with other macromolecules and fine-tune the decoding of mRNAs during translation. Their presence in functionally important regions of tRNA is conserved in all domains of life. However, the identities of many of these modified residues depend much on the phylogeny of organisms the tRNAs are found in, attesting for domain-specific strategies of tRNA maturation. In this work we present a new tool, tRNAmodviz web server (http://genesilico.pl/trnamodviz) for easy comparative analysis and visualization of modification patterns in individual tRNAs, as well as in groups of selected tRNA sequences. We also present results of comparative analysis of tRNA sequences derived from 7 phylogenetically distinct groups of organisms: Gram-negative bacteria, Gram-positive bacteria, cytosol of eukaryotic single cell organisms, Fungi and Metazoa, cytosol of Viridiplantae, mitochondria, plastids and Euryarchaeota. These data update the study conducted 20 y ago with the tRNA sequences available at that time.
Keywords: RNA maturation; comparative analysis; evolution; modified nucleotides; post-transcriptional modification; tRNA; tRNA modifications; tRNA sequence; web server.
Figures


Similar articles
-
tRNAmodpred: A computational method for predicting posttranscriptional modifications in tRNAs.Methods. 2016 Sep 1;107:34-41. doi: 10.1016/j.ymeth.2016.03.013. Epub 2016 Mar 23. Methods. 2016. PMID: 27016142 Free PMC article.
-
tRNA Modification Profiles and Codon-Decoding Strategies in Methanocaldococcus jannaschii.J Bacteriol. 2019 Apr 9;201(9):e00690-18. doi: 10.1128/JB.00690-18. Print 2019 May 1. J Bacteriol. 2019. PMID: 30745370 Free PMC article.
-
Post-Transcriptional Modifications of Conserved Nucleotides in the T-Loop of tRNA: A Tale of Functional Convergent Evolution.Genes (Basel). 2021 Jan 22;12(2):140. doi: 10.3390/genes12020140. Genes (Basel). 2021. PMID: 33499018 Free PMC article. Review.
-
To be or not to be modified: Miscellaneous aspects influencing nucleotide modifications in tRNAs.IUBMB Life. 2019 Aug;71(8):1126-1140. doi: 10.1002/iub.2041. Epub 2019 Apr 1. IUBMB Life. 2019. PMID: 30932315 Free PMC article. Review.
-
Eukaryotic tRNAs fingerprint invertebrates vis-à-vis vertebrates.J Biomol Struct Dyn. 2015;33(10):2104-20. doi: 10.1080/07391102.2014.990925. Epub 2015 Jan 12. J Biomol Struct Dyn. 2015. PMID: 25581620
Cited by
-
Aspergillus fumigatus Elongator complex subunit 3 affects hyphal growth, adhesion and virulence through wobble uridine tRNA modification.PLoS Pathog. 2022 Nov 14;18(11):e1010976. doi: 10.1371/journal.ppat.1010976. eCollection 2022 Nov. PLoS Pathog. 2022. PMID: 36374932 Free PMC article.
-
Changes of the tRNA Modification Pattern during the Development of Dictyostelium discoideum.Noncoding RNA. 2021 May 28;7(2):32. doi: 10.3390/ncrna7020032. Noncoding RNA. 2021. PMID: 34071416 Free PMC article.
-
Combining Nanopore direct RNA sequencing with genetics and mass spectrometry for analysis of T-loop base modifications across 42 yeast tRNA isoacceptors.Nucleic Acids Res. 2024 Oct 28;52(19):12074-12092. doi: 10.1093/nar/gkae796. Nucleic Acids Res. 2024. PMID: 39340295 Free PMC article.
-
Identification of a novel 5-aminomethyl-2-thiouridine methyltransferase in tRNA modification.Nucleic Acids Res. 2023 Feb 28;51(4):1971-1983. doi: 10.1093/nar/gkad048. Nucleic Acids Res. 2023. PMID: 36762482 Free PMC article.
-
Structural Significance of Conformational Preferences and Ribose-Ring-Puckering of Hyper Modified Nucleotide 5'-Monophosphate 2-Methylthio Cyclic N6-Threonylcarbamoyladenosine (p-ms2ct6A) Present at 37th Position in Anticodon Loop of tRNALys.Cell Biochem Biophys. 2022 Dec;80(4):665-680. doi: 10.1007/s12013-022-01086-0. Epub 2022 Aug 15. Cell Biochem Biophys. 2022. PMID: 35965304
References
-
- Motorin Y, Helm M. tRNA stabilization by modified nucleotides. Biochemistry 2010; 49:4934-44; PMID:20459084; http://dx.doi.org/10.1021/bi100408z - DOI - PubMed
-
- Giegé R, Lapointe J. Transfer RNA aminoacylation and modified nucleosides. In: Grosjean H, ed. DNA and RNA Modification Enzymes: Structure, Mechanism, Function and Evolution. Austin, Texas, USA: Landes Bioscience, 2009:476-92
-
- Agris PF. Bringing order to translation: the contributions of transfer RNA anticodon-domain modifications. EMBO Rep 2008; 9:629-35; PMID:18552770; http://dx.doi.org/10.1038/embor.2008.104 - DOI - PMC - PubMed
-
- Grosjean H, de Crecy-Lagard V, Marck C. Deciphering synonymous codons in the three domains of life: co-evolution with specific tRNA modification enzymes. FEBS letters 2010; 584:252-64; PMID:19931533; http://dx.doi.org/10.1016/j.febslet.2009.11.052 - DOI - PubMed
-
- Helm M, Alfonzo JD. Posttranscriptional RNA Modifications: playing metabolic games in a cell's chemical Legoland. Chem Biol 2014; 21:174-85; PMID:24315934; http://dx.doi.org/10.1016/j.chembiol.2013.10.015 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
