Systematic mapping of WNT-FZD protein interactions reveals functional selectivity by distinct WNT-FZD pairs
- PMID: 25605717
- PMCID: PMC4358105
- DOI: 10.1074/jbc.M114.612648
Systematic mapping of WNT-FZD protein interactions reveals functional selectivity by distinct WNT-FZD pairs
Abstract
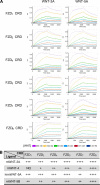
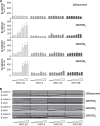
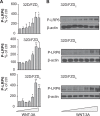
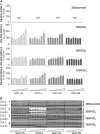
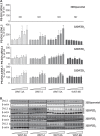
The seven-transmembrane-spanning receptors of the FZD1-10 class are bound and activated by the WNT family of lipoglycoproteins, thereby inducing a complex network of signaling pathways. However, the specificity of the interaction between mammalian WNT and FZD proteins and the subsequent signaling cascade downstream of the different WNT-FZD pairs have not been systematically addressed to date. In this study, we determined the binding affinities of various WNTs for different members of the FZD family by using bio-layer interferometry and characterized their functional selectivity in a cell system. Using purified WNTs, we show that different FZD cysteine-rich domains prefer to bind to distinct WNTs with fast on-rates and slow off-rates. In a 32D cell-based system engineered to overexpress FZD2, FZD4, or FZD5, we found that WNT-3A (but not WNT-4, -5A, or -9B) activated the WNT-β-catenin pathway through FZD2/4/5 as measured by phosphorylation of LRP6 and β-catenin stabilization. Surprisingly, different WNT-FZD pairs showed differential effects on phosphorylation of DVL2 and DVL3, revealing a previously unappreciated DVL isoform selectivity by different WNT-FZD pairs in 32D cells. In summary, we present extensive mapping of WNT-FZD cysteine-rich domain interactions complemented by analysis of WNT-FZD pair functionality in a unique cell system expressing individual FZD isoforms. Differential WNT-FZD binding and selective functional readouts suggest that endogenous WNT ligands evolved with an intrinsic natural bias toward different downstream signaling pathways, a phenomenon that could be of great importance in the design of FZD-targeting drugs.
Keywords: 32D Cells; Disheveled; Frizzled; Functional Selectivity; LDL Receptor-related Protein 6; Myeloid Cell; Receptor; WNT Pathway; WNT Signaling; β-Catenin (B-catenin).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Parathyroid hormone receptor directly interacts with dishevelled to regulate beta-Catenin signaling and osteoclastogenesis.J Biol Chem. 2010 May 7;285(19):14756-63. doi: 10.1074/jbc.M110.102970. Epub 2010 Mar 8. J Biol Chem. 2010. PMID: 20212039 Free PMC article.
-
Assessment of Frizzled 6 membrane mobility by FRAP supports G protein coupling and reveals WNT-Frizzled selectivity.Cell Signal. 2014 Sep;26(9):1943-9. doi: 10.1016/j.cellsig.2014.05.012. Epub 2014 May 27. Cell Signal. 2014. PMID: 24873871
-
β-Catenin-dependent pathway activation by both promiscuous "canonical" WNT3a-, and specific "noncanonical" WNT4- and WNT5a-FZD receptor combinations with strong differences in LRP5 and LRP6 dependency.Cell Signal. 2014 Feb;26(2):260-7. doi: 10.1016/j.cellsig.2013.11.021. Epub 2013 Nov 21. Cell Signal. 2014. PMID: 24269653
-
Frizzleds and WNT/β-catenin signaling--The black box of ligand-receptor selectivity, complex stoichiometry and activation kinetics.Eur J Pharmacol. 2015 Sep 15;763(Pt B):191-5. doi: 10.1016/j.ejphar.2015.05.031. Epub 2015 May 21. Eur J Pharmacol. 2015. PMID: 26003275 Review.
-
Wnt/Frizzled signaling in hepatocellular carcinoma.Front Biosci. 2006 May 1;11:1901-15. doi: 10.2741/1933. Front Biosci. 2006. PMID: 16368566 Review.
Cited by
-
Why Is Wnt/β-Catenin Not Yet Targeted in Routine Cancer Care?Pharmaceuticals (Basel). 2024 Jul 16;17(7):949. doi: 10.3390/ph17070949. Pharmaceuticals (Basel). 2024. PMID: 39065798 Free PMC article. Review.
-
High Canonical Wnt/β-Catenin Activity Sensitizes Murine Hematopoietic Stem and Progenitor Cells to DNA Damage.Stem Cell Rev Rep. 2020 Feb;16(1):212-221. doi: 10.1007/s12015-019-09930-2. Stem Cell Rev Rep. 2020. PMID: 31797147 Free PMC article.
-
Wnt/β-Catenin Signaling as a Molecular Target by Pathogenic Bacteria.Front Immunol. 2019 Sep 27;10:2135. doi: 10.3389/fimmu.2019.02135. eCollection 2019. Front Immunol. 2019. PMID: 31611869 Free PMC article. Review.
-
Anti-tumor activity of a recombinant soluble Fzd7 decoy receptor in human gastric and colon cancer cells.Iran J Basic Med Sci. 2022 Feb;25(2):187-192. doi: 10.22038/IJBMS.2022.61908.13700. Iran J Basic Med Sci. 2022. PMID: 35655594 Free PMC article.
-
Frizzled-5: a high affinity receptor for secreted frizzled-related protein-2 activation of nuclear factor of activated T-cells c3 signaling to promote angiogenesis.Angiogenesis. 2017 Nov;20(4):615-628. doi: 10.1007/s10456-017-9574-5. Epub 2017 Aug 24. Angiogenesis. 2017. PMID: 28840375 Free PMC article.
References
-
- van Amerongen R., Nusse R. (2009) Towards an integrated view of Wnt signaling in development. Development 136, 3205–3214 - PubMed
-
- Schulte G. (2010) International Union of Basic and Clinical Pharmacology. LXXX. The class Frizzled receptors. Pharmacol. Rev. 62, 632–667 - PubMed
-
- Egger-Adam D., Katanaev V. L. (2008) Trimeric G protein-dependent signaling by Frizzled receptors in animal development. Front. Biosci. 13, 4740–4755 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

