Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry
- PMID: 25604608
- PMCID: PMC4365093
- DOI: 10.1208/s12248-014-9710-8
Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry
Abstract
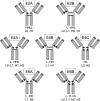
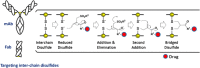
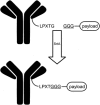
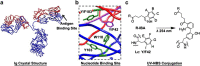
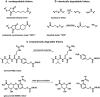
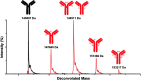
Antibody drug conjugates (ADCs) have emerged as an important pharmaceutical class of drugs designed to harness the specificity of antibodies with the potency of small molecule therapeutics. The three main components of ADCs are the antibody, the linker, and the payload; the majority of early work focused intensely on improving the functionality of these pieces. Recently, considerable attention has been focused on developing methods to control the site and number of linker/drug conjugated to the antibody, with the aim of producing more homogenous ADCs. In this article, we review popular conjugation methods and highlight recent approaches including "click" conjugation and enzymatic ligation. We discuss current linker technology, contrasting the characteristics of cleavable and non-cleavable linkers, and summarize the essential properties of ADC payload, centering on chemotherapeutics. In addition, we report on the progress in characterizing to determine physicochemical properties and on advances in purifying to obtain homogenous products. Establishing a set of selection and analytical criteria will facilitate the translation of novel ADCs and ensure the production of effective biosimilars.
Figures



Similar articles
-
Cleavable linkers in antibody-drug conjugates.Chem Soc Rev. 2019 Aug 12;48(16):4361-4374. doi: 10.1039/c8cs00676h. Chem Soc Rev. 2019. PMID: 31294429 Review.
-
Physical and Chemical Stability of Antibody Drug Conjugates: Current Status.J Pharm Sci. 2016 Feb;105(2):391-397. doi: 10.1016/j.xphs.2015.11.037. J Pharm Sci. 2016. PMID: 26869406 Review.
-
Antibody-drug conjugates: recent advances in conjugation and linker chemistries.Protein Cell. 2018 Jan;9(1):33-46. doi: 10.1007/s13238-016-0323-0. Epub 2016 Oct 14. Protein Cell. 2018. PMID: 27743348 Free PMC article. Review.
-
[Novel Chemical Linkers for Next-generation Antibody-drug Conjugates(ADCs)].Yakugaku Zasshi. 2019;139(2):209-219. doi: 10.1248/yakushi.18-00169-3. Yakugaku Zasshi. 2019. PMID: 30713230 Review. Japanese.
-
Exploring the effects of linker composition on site-specifically modified antibody-drug conjugates.Eur J Med Chem. 2014 Dec 17;88:3-9. doi: 10.1016/j.ejmech.2014.08.062. Epub 2014 Aug 23. Eur J Med Chem. 2014. PMID: 25176286
Cited by
-
Antibody-Drug Conjugates in Gynecologic Cancers.Curr Treat Options Oncol. 2024 Jan;25(1):1-19. doi: 10.1007/s11864-023-01166-0. Epub 2024 Jan 3. Curr Treat Options Oncol. 2024. PMID: 38172449 Review.
-
Linkers: An Assurance for Controlled Delivery of Antibody-Drug Conjugate.Pharmaceutics. 2022 Feb 11;14(2):396. doi: 10.3390/pharmaceutics14020396. Pharmaceutics. 2022. PMID: 35214128 Free PMC article. Review.
-
EpCAM- and EGFR-Specific Antibody Drug Conjugates for Triple-Negative Breast Cancer Treatment.Int J Mol Sci. 2022 May 30;23(11):6122. doi: 10.3390/ijms23116122. Int J Mol Sci. 2022. PMID: 35682800 Free PMC article.
-
Importance and Considerations of Antibody Engineering in Antibody-Drug Conjugates Development from a Clinical Pharmacologist's Perspective.Antibodies (Basel). 2021 Jul 26;10(3):30. doi: 10.3390/antib10030030. Antibodies (Basel). 2021. PMID: 34449544 Free PMC article. Review.
-
The landscape of new drugs in lymphoma.Nat Rev Clin Oncol. 2017 Jun;14(6):335-346. doi: 10.1038/nrclinonc.2016.205. Epub 2016 Dec 29. Nat Rev Clin Oncol. 2017. PMID: 28031560 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

