Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins
- PMID: 25603345
- PMCID: PMC4306949
- DOI: 10.3390/md13010509
Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins
Abstract
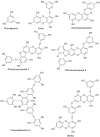
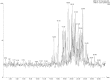
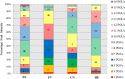
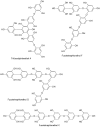
Phlorotannins are a group of complex polymers of phloroglucinol (1,3,5-trihydroxybenzene) unique to macroalgae. These phenolic compounds are integral structural components of the cell wall in brown algae, but also play many secondary ecological roles such as protection from UV radiation and defense against grazing. This study employed Ultra Performance Liquid Chromatography (UPLC) with tandem mass spectrometry to investigate isomeric complexity and observed differences in phlorotannins derived from macroalgae harvested off the Irish coast (Fucus serratus, Fucus vesiculosus, Himanthalia elongata and Cystoseira nodicaulis). Antioxidant activity and total phenolic content assays were used as an index for producing phlorotannin fractions, enriched using molecular weight cut-off dialysis with subsequent flash chromatography to profile phlorotannin isomers in these macroalgae. These fractions were profiled using UPLC-MS with multiple reaction monitoring (MRM) and the level of isomerization for specific molecular weight phlorotannins between 3 and 16 monomers were determined. The majority of the low molecular weight (LMW) phlorotannins were found to have a molecular weight range equivalent to 4-12 monomers of phloroglucinol. The level of isomerization within the individual macroalgal species differed, resulting in substantially different numbers of phlorotannin isomers for particular molecular weights. F. vesiculosus had the highest number of isomers of 61 at one specific molecular mass, corresponding to 12 phloroglucinol units (PGUs). These results highlight the complex nature of these extracts and emphasize the challenges involved in structural elucidation of these compounds.
Figures


Similar articles
-
Profiling phlorotannins in brown macroalgae by liquid chromatography-high resolution mass spectrometry.Phytochem Anal. 2012 Sep-Oct;23(5):547-53. doi: 10.1002/pca.2354. Epub 2012 Mar 2. Phytochem Anal. 2012. PMID: 22383068
-
Optimization of Phlorotannins Extraction from Fucus vesiculosus and Evaluation of Their Potential to Prevent Metabolic Disorders.Mar Drugs. 2019 Mar 8;17(3):162. doi: 10.3390/md17030162. Mar Drugs. 2019. PMID: 30857204 Free PMC article.
-
Identification of Phlorotannins in the Brown Algae, Saccharina latissima and Ascophyllum nodosum by Ultra-High-Performance Liquid Chromatography Coupled to High-Resolution Tandem Mass Spectrometry.Molecules. 2020 Dec 23;26(1):43. doi: 10.3390/molecules26010043. Molecules. 2020. PMID: 33374856 Free PMC article.
-
The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies.Biomolecules. 2019 Dec 9;9(12):847. doi: 10.3390/biom9120847. Biomolecules. 2019. PMID: 31835386 Free PMC article. Review.
-
Call the Eckols: Present and Future Potential Cancer Therapies.Mar Drugs. 2022 Jun 9;20(6):387. doi: 10.3390/md20060387. Mar Drugs. 2022. PMID: 35736190 Free PMC article. Review.
Cited by
-
Metabolite Profiling of Macroalgae: Biosynthesis and Beneficial Biological Properties of Active Compounds.Mar Drugs. 2024 Oct 19;22(10):478. doi: 10.3390/md22100478. Mar Drugs. 2024. PMID: 39452886 Free PMC article. Review.
-
Investigating the Anti-Inflammatory Activity of Various Brown Algae Species.Mar Drugs. 2024 Oct 5;22(10):457. doi: 10.3390/md22100457. Mar Drugs. 2024. PMID: 39452865 Free PMC article. Review.
-
Integrated Valorization of Fucus spiralis Alga: Polysaccharides and Bioactives for Edible Films and Residues as Biostimulants.Foods. 2024 Sep 17;13(18):2938. doi: 10.3390/foods13182938. Foods. 2024. PMID: 39335867 Free PMC article.
-
Fucus vesiculosus-Rich Extracts as Potential Functional Food Ingredients: A Holistic Extraction Approach.Foods. 2024 Feb 9;13(4):540. doi: 10.3390/foods13040540. Foods. 2024. PMID: 38397517 Free PMC article.
-
Phlorotannins: Novel Orally Administrated Bioactive Compounds That Induce Mitochondrial Dysfunction and Oxidative Stress in Cancer.Antioxidants (Basel). 2023 Sep 7;12(9):1734. doi: 10.3390/antiox12091734. Antioxidants (Basel). 2023. PMID: 37760037 Free PMC article. Review.
References
-
- Lopes G., Sousa C., Silva L.R., Pinto E., Andrade P.B., Bernardo J., Mouga T., Valentão P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS One. 2012;7:e31145. doi: 10.1371/journal.pone.0031145. - DOI - PMC - PubMed
-
- Li Y.X., Wijeseker I., Li Y., Kim S.K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011;46:2219–2224. doi: 10.1016/j.procbio.2011.09.015. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

