Calcipotriol delivery into the skin as emulgel for effective permeation
- PMID: 25561873
- PMCID: PMC4281593
- DOI: 10.1016/j.jsps.2014.02.007
Calcipotriol delivery into the skin as emulgel for effective permeation
Abstract
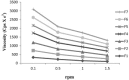
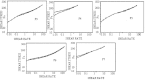
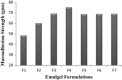
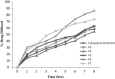
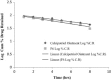
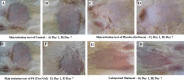
The objective of this work is to formulate and evaluate an emulgel containing calcipotriol for treatment of psoriasis. Emulgels have emerged as a promising drug delivery system for the delivery of hydrophobic drugs. Isopropyl alcohol and polyethylene glycol have been employed as permeation enhancers. Formulation chart is made with seven formulations, evaluated for physical parameters, drug content, viscosity, thixotropy, spreadability, extrudability, mucoadhesion, diffusion studies, skin irritation test along with short term stability studies. Carbopolis is reported to have a direct influence on appearance and viscosity of final formulation. The photomicroscopic evaluations showed the presence of spherical globules in size range of 10-15 μm. Rheograms revealed that all the formulations exhibited pseudoplastic flow. Optimized formulation (F6) had shown 86.42 ± 2.0% drug release at the end of 8 h study. The release rate through dialysis membrane and rat skin is higher when compared to commercial calcipotriol ointment. Hence it is concluded that calcipotriol can be delivered topically with enhanced penetration properties when formulated as emulgel.
Keywords: Calcipotriol; Carbopol; Emulgel; PEG; Topical delivery.
Figures


Similar articles
-
Emulgel for improved topical delivery of Tretinoin: Formulation design and characterization.Ann Pharm Fr. 2022 Mar;80(2):157-168. doi: 10.1016/j.pharma.2021.05.004. Epub 2021 May 21. Ann Pharm Fr. 2022. PMID: 34029557
-
Development of an Aloe vera-based Emulgel for the Topical Delivery of Desoximetasone.Turk J Pharm Sci. 2021 Sep 1;18(4):465-475. doi: 10.4274/tjps.galenos.2020.33239. Turk J Pharm Sci. 2021. PMID: 34496553 Free PMC article.
-
Topical drug delivery by Sepineo P600 emulgel: Relationship between rheology, physical stability, and formulation performance.Int J Pharm. 2024 Jun 10;658:124210. doi: 10.1016/j.ijpharm.2024.124210. Epub 2024 May 7. Int J Pharm. 2024. PMID: 38718972
-
Recent expansions in an emergent novel drug delivery technology: Emulgel.J Control Release. 2013 Oct 28;171(2):122-32. doi: 10.1016/j.jconrel.2013.06.030. Epub 2013 Jul 2. J Control Release. 2013. PMID: 23831051 Review.
-
Cellulose derivatives and natural gums as gelling agents for preparation of emulgel-based dosage forms: A brief review.Int J Biol Macromol. 2023 Jun 30;241:124538. doi: 10.1016/j.ijbiomac.2023.124538. Epub 2023 Apr 19. Int J Biol Macromol. 2023. PMID: 37085064 Review.
Cited by
-
Polymeric Gels and Their Application in the Treatment of Psoriasis Vulgaris: A Review.Int J Mol Sci. 2021 May 12;22(10):5124. doi: 10.3390/ijms22105124. Int J Mol Sci. 2021. PMID: 34066105 Free PMC article. Review.
-
Original Research Article (Experimental): Design and Development of Unani Emulgel for Vitiligo.J Ayurveda Integr Med. 2020 Jul-Sep;11(3):199-205. doi: 10.1016/j.jaim.2018.01.006. Epub 2018 Nov 17. J Ayurveda Integr Med. 2020. PMID: 30459078 Free PMC article.
-
Artemisinin emulgel ameliorates cartilage degradation in knee osteoarthritis: in vitro and in vivo studies.Ther Deliv. 2024;15(12):939-955. doi: 10.1080/20415990.2024.2418281. Epub 2024 Nov 6. Ther Deliv. 2024. PMID: 39503537
-
Meloxicam emulgels for topical management of rheumatism: Formulation development, in vitro and in vivo characterization.Saudi Pharm J. 2021 Apr;29(4):351-360. doi: 10.1016/j.jsps.2021.03.005. Epub 2021 Mar 23. Saudi Pharm J. 2021. PMID: 33994830 Free PMC article.
-
Enhanced Corneal Penetration of a Poorly Permeable Drug Using Bioadhesive Multiple Microemulsion Technology.Pharmaceutics. 2020 Jul 26;12(8):704. doi: 10.3390/pharmaceutics12080704. Pharmaceutics. 2020. PMID: 32722550 Free PMC article.
References
-
- Pradhan M., Singh D., Singh M.R. Novel colloidal carriers for psoriasis: current issues, mechanistic insight and novel delivery approaches. J. Control. Release. 2013;170(3):380–395. - PubMed
-
- Gupta A., Mishra A.K., Singh A.K., Bansal P., Gupta V. Formulation and evaluation of topical gel of diclofenac sodium using different polymers. Drug Invent. Today. 2010;2:250–253.
-
- Rieger M.M. Emulsions. In: Lachman L., Lieberman H.A., Kanig J.L., editors. The Theory and Practice of Industrial Pharmacy. Third ed. Lea and Febiger; Philadelphia, PA: 1986. pp. 502–533.
LinkOut - more resources
Full Text Sources
Other Literature Sources

