Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo
- PMID: 25539810
- PMCID: PMC4380504
- DOI: 10.4049/jimmunol.1401686
Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo
Abstract
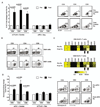
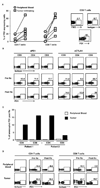
Combination therapy concurrently targeting PD-1 and CTLA-4 immune checkpoints leads to remarkable antitumor effects. Although both PD-1 and CTLA-4 dampen the T cell activation, the in vivo effects of these drugs in humans remain to be clearly defined. To better understand biologic effects of therapy, we analyzed blood/tumor tissue from 45 patients undergoing single or combination immune checkpoint blockade. We show that blockade of CTLA-4, PD-1, or combination of the two leads to distinct genomic and functional signatures in vivo in purified human T cells and monocytes. Therapy-induced changes are more prominent in T cells than in monocytes and involve largely nonoverlapping changes in coding genes, including alternatively spliced transcripts and noncoding RNAs. Pathway analysis revealed that CTLA-4 blockade induces a proliferative signature predominantly in a subset of transitional memory T cells, whereas PD-1 blockade instead leads to changes in genes implicated in cytolysis and NK cell function. Combination blockade leads to nonoverlapping changes in gene expression, including proliferation-associated and chemokine genes. These therapies also have differential effects on plasma levels of CXCL10, soluble IL-2R, and IL-1α. Importantly, PD-1 receptor occupancy following anti-PD-1 therapy may be incomplete in the tumor T cells even in the setting of complete receptor occupancy in circulating T cells. These data demonstrate that, despite shared property of checkpoint blockade, Abs against PD-1, CTLA-4 alone, or in combination have distinct immunologic effects in vivo. Improved understanding of pharmacodynamic effects of these agents in patients will support rational development of immune-based combinations against cancer.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures

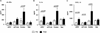
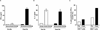
Similar articles
-
Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs.Clin Cancer Res. 2013 Oct 15;19(20):5626-35. doi: 10.1158/1078-0432.CCR-13-0545. Epub 2013 Aug 27. Clin Cancer Res. 2013. PMID: 23983257
-
Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade.Cancer Discov. 2016 Aug;6(8):827-37. doi: 10.1158/2159-8290.CD-15-1545. Epub 2016 Jun 14. Cancer Discov. 2016. PMID: 27301722 Free PMC article.
-
Timing of PD-1 Blockade Is Critical to Effective Combination Immunotherapy with Anti-OX40.Clin Cancer Res. 2017 Oct 15;23(20):6165-6177. doi: 10.1158/1078-0432.CCR-16-2677. Epub 2017 Aug 28. Clin Cancer Res. 2017. PMID: 28855348 Free PMC article.
-
Immune Checkpoint Blockade in Cancer Therapy.J Clin Oncol. 2015 Jun 10;33(17):1974-82. doi: 10.1200/JCO.2014.59.4358. Epub 2015 Jan 20. J Clin Oncol. 2015. PMID: 25605845 Free PMC article. Review.
-
Immune checkpoint protein inhibition for cancer: preclinical justification for CTLA-4 and PD-1 blockade and new combinations.Semin Oncol. 2015 Jun;42(3):363-77. doi: 10.1053/j.seminoncol.2015.02.015. Epub 2015 Feb 16. Semin Oncol. 2015. PMID: 25965355 Review.
Cited by
-
A phase Ib study evaluating the safety and efficacy of IBI310 plus sintilimab in patients with advanced non-small-cell lung cancer who have progressed after anti-PD-1/L1 therapy.Cancer Med. 2024 Feb;13(3):e6855. doi: 10.1002/cam4.6855. Epub 2024 Jan 12. Cancer Med. 2024. PMID: 38214075 Free PMC article. Clinical Trial.
-
ABC transporters and NR4A1 identify a quiescent subset of tissue-resident memory T cells.J Clin Invest. 2016 Oct 3;126(10):3905-3916. doi: 10.1172/JCI85329. Epub 2016 Sep 12. J Clin Invest. 2016. PMID: 27617863 Free PMC article.
-
Ipilimumab reshapes T cell memory subsets in melanoma patients with clinical response.Oncoimmunology. 2016 Feb 18;5(7):1136045. doi: 10.1080/2162402X.2015.1136045. eCollection 2016 Jul. Oncoimmunology. 2016. PMID: 27622012 Free PMC article.
-
Targeting the Immune Microenvironment in the Treatment of Head and Neck Squamous Cell Carcinoma.Front Oncol. 2019 Oct 15;9:1084. doi: 10.3389/fonc.2019.01084. eCollection 2019. Front Oncol. 2019. PMID: 31681613 Free PMC article. Review.
-
Role of Tissue-Resident Memory in Intra-Tumor Heterogeneity and Response to Immune Checkpoint Blockade.Front Immunol. 2018 Jul 16;9:1655. doi: 10.3389/fimmu.2018.01655. eCollection 2018. Front Immunol. 2018. PMID: 30061902 Free PMC article. Review.
References
-
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. - PMC - PubMed
-
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

