Myeloid-derived suppressor activity is mediated by monocytic lineages maintained by continuous inhibition of extrinsic and intrinsic death pathways
- PMID: 25500368
- PMCID: PMC4272664
- DOI: 10.1016/j.immuni.2014.10.020
Myeloid-derived suppressor activity is mediated by monocytic lineages maintained by continuous inhibition of extrinsic and intrinsic death pathways
Abstract
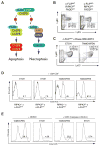
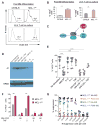
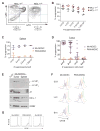
Nonresolving inflammation expands a heterogeneous population of myeloid suppressor cells capable of inhibiting T cell function. This heterogeneity has confounded the functional dissection of individual myeloid subpopulations and presents an obstacle for antitumor immunity and immunotherapy. Using genetic manipulation of cell death pathways, we found the monocytic suppressor-cell subset, but not the granulocytic subset, requires continuous c-FLIP expression to prevent caspase-8-dependent, RIPK3-independent cell death. Development of the granulocyte subset requires MCL-1-mediated control of the intrinsic mitochondrial death pathway. Monocytic suppressors tolerate the absence of MCL-1 provided cytokines increase expression of the MCL-1-related protein A1. Monocytic suppressors mediate T cell suppression, whereas their granulocytic counterparts lack suppressive function. The loss of the granulocytic subset via conditional MCL-1 deletion did not alter tumor incidence implicating the monocytic compartment as the functionally immunosuppressive subset in vivo. Thus, death pathway modulation defines the development, survival, and function of myeloid suppressor cells.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Role of death receptor and mitochondrial pathways in conventional chemotherapy drug induction of apoptosis.Cell Signal. 2006 Sep;18(9):1528-35. doi: 10.1016/j.cellsig.2005.12.004. Epub 2006 Jan 25. Cell Signal. 2006. PMID: 16442262
-
c-FLIP and the NOXA/Mcl-1 axis participate in the synergistic effect of pemetrexed plus cisplatin in human choroidal melanoma cells.PLoS One. 2017 Sep 1;12(9):e0184135. doi: 10.1371/journal.pone.0184135. eCollection 2017. PLoS One. 2017. PMID: 28863158 Free PMC article.
-
Deregulated c-Myc prematurely recruits both Type I and II CD95/Fas apoptotic pathways associated with terminal myeloid differentiation.Oncogene. 2002 Feb 28;21(10):1600-10. doi: 10.1038/sj.onc.1205231. Oncogene. 2002. PMID: 11896589
-
Apoptosis and autophagy in the regulation of T lymphocyte function.Immunol Res. 2011 Apr;49(1-3):70-86. doi: 10.1007/s12026-010-8195-5. Immunol Res. 2011. PMID: 21128005 Free PMC article. Review.
-
The inflammatory role of phagocyte apoptotic pathways in rheumatic diseases.Nat Rev Rheumatol. 2016 Aug 23;12(9):543-58. doi: 10.1038/nrrheum.2016.132. Nat Rev Rheumatol. 2016. PMID: 27549026 Free PMC article. Review.
Cited by
-
Sunitinib enhances the antitumor responses of agonistic CD40-antibody by reducing MDSCs and synergistically improving endothelial activation and T-cell recruitment.Oncotarget. 2016 Jul 1;7(31):50277-50289. doi: 10.18632/oncotarget.10364. Oncotarget. 2016. PMID: 27385210 Free PMC article.
-
The Tumor Microenvironment in Colorectal Cancer Therapy.Cancers (Basel). 2019 Aug 14;11(8):1172. doi: 10.3390/cancers11081172. Cancers (Basel). 2019. PMID: 31416205 Free PMC article. Review.
-
5-Fluorouracil Suppresses Colon Tumor through Activating the p53-Fas Pathway to Sensitize Myeloid-Derived Suppressor Cells to FasL+ Cytotoxic T Lymphocyte Cytotoxicity.Cancers (Basel). 2023 Mar 2;15(5):1563. doi: 10.3390/cancers15051563. Cancers (Basel). 2023. PMID: 36900354 Free PMC article.
-
The role of myeloid-derived suppressor cells in liver cancer.Discov Oncol. 2023 May 23;14(1):77. doi: 10.1007/s12672-023-00681-8. Discov Oncol. 2023. PMID: 37217620 Free PMC article. Review.
-
Proteomic signatures of myeloid derived suppressor cells from liver and lung metastases reveal functional divergence and potential therapeutic targets.Cell Death Discov. 2021 Sep 4;7(1):232. doi: 10.1038/s41420-021-00621-x. Cell Death Discov. 2021. PMID: 34482371 Free PMC article.
References
-
- Dolcetti L, Peranzoni E, Bronte V. Measurement of myeloid cell immune suppressive activity. Curr Protoc Immunol. 2010;Chapter 14(Unit 14–17) - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

