HIV-1 Tat protein induces glial cell autophagy through enhancement of BAG3 protein levels
- PMID: 25483098
- PMCID: PMC4614156
- DOI: 10.4161/15384101.2014.952959
HIV-1 Tat protein induces glial cell autophagy through enhancement of BAG3 protein levels
Abstract
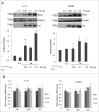
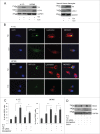
BAG3 protein has been described as an anti-apoptotic and pro-autophagic factor in several neoplastic and normal cells. We previously demonstrated that BAG3 expression is elevated upon HIV-1 infection of glial and T lymphocyte cells. Among HIV-1 proteins, Tat is highly involved in regulating host cell response to viral infection. Therefore, we investigated the possible role of Tat protein in modulating BAG3 protein levels and the autophagic process itself. In this report, we show that transfection with Tat raises BAG3 levels in glioblastoma cells. Moreover, BAG3 silencing results in highly reducing Tat- induced levels of LC3-II and increasing the appearance of sub G0/G1 apoptotic cells, in keeping with the reported role of BAG3 in modulating the autophagy/apoptosis balance. These results demonstrate for the first time that Tat protein is able to stimulate autophagy through increasing BAG3 levels in human glial cells.
Keywords: BAG3; BAG3, Bcl-2-Associated Athanogene 3; HIV-1; LC3, microtubule-associated protein 1 light chain 3; Tat; Tat, Trans-Activator of Transcription;; autophagy; glioblastoma.
Figures
Similar articles
-
HIV-1 Tat increases BAG3 via NF-κB signaling to induce autophagy during HIV-associated neurocognitive disorder.Cell Cycle. 2018;17(13):1614-1623. doi: 10.1080/15384101.2018.1480219. Epub 2018 Aug 21. Cell Cycle. 2018. PMID: 29962275 Free PMC article.
-
Perturbation of synapsins homeostasis through HIV-1 Tat-mediated suppression of BAG3 in primary neuronal cells.Cell Death Dis. 2019 Jun 17;10(7):473. doi: 10.1038/s41419-019-1702-2. Cell Death Dis. 2019. PMID: 31209204 Free PMC article.
-
Bag3-induced autophagy is associated with degradation of JCV oncoprotein, T-Ag.PLoS One. 2012;7(9):e45000. doi: 10.1371/journal.pone.0045000. Epub 2012 Sep 12. PLoS One. 2012. PMID: 22984599 Free PMC article.
-
Breaking BAG: The Co-Chaperone BAG3 in Health and Disease.Trends Pharmacol Sci. 2016 Aug;37(8):672-688. doi: 10.1016/j.tips.2016.04.007. Epub 2016 May 6. Trends Pharmacol Sci. 2016. PMID: 27162137 Review.
-
At the Crossroads of Apoptosis and Autophagy: Multiple Roles of the Co-Chaperone BAG3 in Stress and Therapy Resistance of Cancer.Cells. 2020 Feb 28;9(3):574. doi: 10.3390/cells9030574. Cells. 2020. PMID: 32121220 Free PMC article. Review.
Cited by
-
Autophagy Intertwines with Different Diseases-Recent Strategies for Therapeutic Approaches.Diseases. 2019 Feb 1;7(1):15. doi: 10.3390/diseases7010015. Diseases. 2019. PMID: 30717078 Free PMC article. Review.
-
The anticancer drug sunitinib promotes autophagyand protects from neurotoxicity in an HIV-1 Tat model of neurodegeneration.J Neurovirol. 2017 Apr;23(2):290-303. doi: 10.1007/s13365-016-0502-z. Epub 2017 Jan 19. J Neurovirol. 2017. PMID: 28105557 Free PMC article.
-
Does HIV infection contribute to increased beta-amyloid synthesis and plaque formation leading to neurodegeneration and Alzheimer's disease?J Neurovirol. 2019 Oct;25(5):634-647. doi: 10.1007/s13365-019-00732-3. Epub 2019 Mar 13. J Neurovirol. 2019. PMID: 30868421 Review.
-
Regulation of NADPH oxidases in skeletal muscle.Free Radic Biol Med. 2016 Sep;98:18-28. doi: 10.1016/j.freeradbiomed.2016.05.011. Epub 2016 May 13. Free Radic Biol Med. 2016. PMID: 27184955 Free PMC article. Review.
-
Virus, Exosome, and MicroRNA: New Insights into Autophagy.Adv Exp Med Biol. 2022;1401:97-162. doi: 10.1007/5584_2022_715. Adv Exp Med Biol. 2022. PMID: 35781219
References
-
- Kramer-Hämmerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res 2005; 11:194-213; PMID:15885841; http://dx.doi.org/10.1016/j.virusres.2005.04.009 - DOI - PubMed
-
- Rubinstein AD, Kimchi A. Life in the balance—a mechanistic view of the crosstalk between autophagy and apoptosis. J Cell Sci 2012; 125:5259-68; PMID:23377657; http://dx.doi.org/10.1242/jcs.115865 - DOI - PubMed
-
- Behl C. BAG3 and friends: co-chaperones in selective autophagy during aging and disease. Autophagy 2011; 7:795-8; PMID:21681022; http://dx.doi.org/10.4161/auto.7.7.15844 - DOI - PubMed
-
- Rosati A, Graziano V, De Laurenzi V, Pascale M, Turco MC. BAG3: a multifaceted protein that regulates major cell pathways. Cell Death Dis 2011; 2:e141; PMID:21472004; http://dx.doi.org/10.1038/cddis.2011.24 - DOI - PMC - PubMed
-
- Ulbricht A, Höhfeld J. Tension- induced autophagy: may the chaperone be with you. Autophagy 2013; 9:920-2; PMID:23518596; http://dx.doi.org/10.4161/auto.24213 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
