Development of covalent inhibitors that can overcome resistance to first-generation FGFR kinase inhibitors
- PMID: 25349422
- PMCID: PMC4234547
- DOI: 10.1073/pnas.1403438111
Development of covalent inhibitors that can overcome resistance to first-generation FGFR kinase inhibitors
Abstract
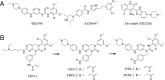
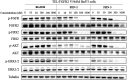
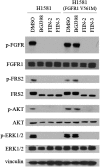
The human FGF receptors (FGFRs) play critical roles in various human cancers, and several FGFR inhibitors are currently under clinical investigation. Resistance usually results from selection for mutant kinases that are impervious to the action of the drug or from up-regulation of compensatory signaling pathways. Preclinical studies have demonstrated that resistance to FGFR inhibitors can be acquired through mutations in the FGFR gatekeeper residue, as clinically observed for FGFR4 in embryonal rhabdomyosarcoma and neuroendocrine breast carcinomas. Here we report on the use of a structure-based drug design to develop two selective, next-generation covalent FGFR inhibitors, the FGFR irreversible inhibitors 2 (FIIN-2) and 3 (FIIN-3). To our knowledge, FIIN-2 and FIIN-3 are the first inhibitors that can potently inhibit the proliferation of cells dependent upon the gatekeeper mutants of FGFR1 or FGFR2, which confer resistance to first-generation clinical FGFR inhibitors such as NVP-BGJ398 and AZD4547. Because of the conformational flexibility of the reactive acrylamide substituent, FIIN-3 has the unprecedented ability to inhibit both the EGF receptor (EGFR) and FGFR covalently by targeting two distinct cysteine residues. We report the cocrystal structure of FGFR4 with FIIN-2, which unexpectedly exhibits a "DFG-out" covalent binding mode. The structural basis for dual FGFR and EGFR targeting by FIIN3 also is illustrated by crystal structures of FIIN-3 bound with FGFR4 V550L and EGFR L858R. These results have important implications for the design of covalent FGFR inhibitors that can overcome clinical resistance and provide the first example, to our knowledge, of a kinase inhibitor that covalently targets cysteines located in different positions within the ATP-binding pocket.
Keywords: cancer drug resistance; drug discovery; kinase inhibitor; structure-based drug design.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
DFG-out mode of inhibition by an irreversible type-1 inhibitor capable of overcoming gate-keeper mutations in FGF receptors.ACS Chem Biol. 2015 Jan 16;10(1):299-309. doi: 10.1021/cb500674s. Epub 2014 Oct 27. ACS Chem Biol. 2015. PMID: 25317566 Free PMC article.
-
Futibatinib Is a Novel Irreversible FGFR 1-4 Inhibitor That Shows Selective Antitumor Activity against FGFR-Deregulated Tumors.Cancer Res. 2020 Nov 15;80(22):4986-4997. doi: 10.1158/0008-5472.CAN-19-2568. Epub 2020 Sep 24. Cancer Res. 2020. PMID: 32973082
-
Probing Dual Covalent Irreversible Inhibition of EGFR/FGFR4 by Electrophilic-Based Natural Compounds to Overcome Resistance and Enhance Combination Therapeutic Potentials and Management of Hepatocellular Carcinoma (HCC).Protein J. 2024 Aug;43(4):793-804. doi: 10.1007/s10930-024-10211-2. Epub 2024 Jul 9. Protein J. 2024. PMID: 38981944
-
FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review).Int J Mol Med. 2016 Jul;38(1):3-15. doi: 10.3892/ijmm.2016.2620. Epub 2016 May 31. Int J Mol Med. 2016. PMID: 27245147 Free PMC article. Review.
-
Genomic aberrations in the FGFR pathway: opportunities for targeted therapies in solid tumors.Ann Oncol. 2014 Mar;25(3):552-563. doi: 10.1093/annonc/mdt419. Epub 2013 Nov 20. Ann Oncol. 2014. PMID: 24265351 Free PMC article. Review.
Cited by
-
Targeting the cancer cells and cancer-associated fibroblasts with next-generation FGFR inhibitors in prostate cancer co-culture models.Cancer Med. 2024 Sep;13(18):e70240. doi: 10.1002/cam4.70240. Cancer Med. 2024. PMID: 39300962 Free PMC article.
-
Novel Regulatory Factors and Small-Molecule Inhibitors of FGFR4 in Cancer.Front Pharmacol. 2021 Apr 26;12:633453. doi: 10.3389/fphar.2021.633453. eCollection 2021. Front Pharmacol. 2021. PMID: 33981224 Free PMC article. Review.
-
The FGFR1 V561M Gatekeeper Mutation Drives AZD4547 Resistance through STAT3 Activation and EMT.Mol Cancer Res. 2019 Feb;17(2):532-543. doi: 10.1158/1541-7786.MCR-18-0429. Epub 2018 Sep 26. Mol Cancer Res. 2019. PMID: 30257990 Free PMC article.
-
Advances in covalent drug discovery.Nat Rev Drug Discov. 2022 Dec;21(12):881-898. doi: 10.1038/s41573-022-00542-z. Epub 2022 Aug 25. Nat Rev Drug Discov. 2022. PMID: 36008483 Free PMC article. Review.
-
Targeting FGFR for cancer therapy.J Hematol Oncol. 2024 Jun 3;17(1):39. doi: 10.1186/s13045-024-01558-1. J Hematol Oncol. 2024. PMID: 38831455 Free PMC article. Review.
References
-
- Breccia M, Alimena G. Nilotinib: A second-generation tyrosine kinase inhibitor for chronic myeloid leukemia. Leuk Res. 2010;34(2):129–134. - PubMed
-
- O’Hare T, et al. Inhibition of wild-type and mutant Bcr-Abl by AP23464, a potent ATP-based oncogenic protein kinase inhibitor: Implications for CML. Blood. 2004;104(8):2532–2539. - PubMed
-
- Solca F, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343(2):342–350. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

