Exploitation of the complement system by oncogenic Kaposi's sarcoma-associated herpesvirus for cell survival and persistent infection
- PMID: 25254972
- PMCID: PMC4177982
- DOI: 10.1371/journal.ppat.1004412
Exploitation of the complement system by oncogenic Kaposi's sarcoma-associated herpesvirus for cell survival and persistent infection
Abstract
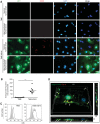
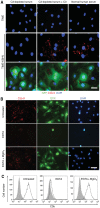
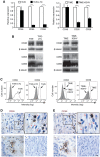
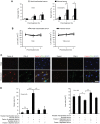
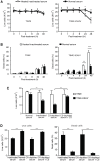
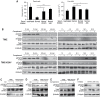
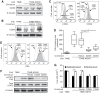
During evolution, herpesviruses have developed numerous, and often very ingenious, strategies to counteract efficient host immunity. Specifically, Kaposi's sarcoma-associated herpesvirus (KSHV) eludes host immunity by undergoing a dormant stage, called latency wherein it expresses a minimal number of viral proteins to evade host immune activation. Here, we show that during latency, KSHV hijacks the complement pathway to promote cell survival. We detected strong deposition of complement membrane attack complex C5b-9 and the complement component C3 activated product C3b on Kaposi's sarcoma spindle tumor cells, and on human endothelial cells latently infected by KSHV, TIME-KSHV and TIVE-LTC, but not on their respective uninfected control cells, TIME and TIVE. We further showed that complement activation in latently KSHV-infected cells was mediated by the alternative complement pathway through down-regulation of cell surface complement regulatory proteins CD55 and CD59. Interestingly, complement activation caused minimal cell death but promoted the survival of latently KSHV-infected cells grown in medium depleted of growth factors. We found that complement activation increased STAT3 tyrosine phosphorylation (Y705) of KSHV-infected cells, which was required for the enhanced cell survival. Furthermore, overexpression of either CD55 or CD59 in latently KSHV-infected cells was sufficient to inhibit complement activation, prevent STAT3 Y705 phosphorylation and abolish the enhanced survival of cells cultured in growth factor-depleted condition. Together, these results demonstrate a novel mechanism by which an oncogenic virus subverts and exploits the host innate immune system to promote viral persistent infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Kaposi's sarcoma associated herpes virus (KSHV) induced COX-2: a key factor in latency, inflammation, angiogenesis, cell survival and invasion.PLoS Pathog. 2010 Feb 12;6(2):e1000777. doi: 10.1371/journal.ppat.1000777. PLoS Pathog. 2010. PMID: 20169190 Free PMC article.
-
Kaposi's sarcoma herpesvirus microRNAs induce metabolic transformation of infected cells.PLoS Pathog. 2014 Sep 25;10(9):e1004400. doi: 10.1371/journal.ppat.1004400. eCollection 2014 Sep. PLoS Pathog. 2014. PMID: 25255370 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus Infection Induces the Expression of Neuroendocrine Genes in Endothelial Cells.J Virol. 2020 Mar 31;94(8):e01692-19. doi: 10.1128/JVI.01692-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31969437 Free PMC article.
-
Pathological Features of Kaposi's Sarcoma-Associated Herpesvirus Infection.Adv Exp Med Biol. 2018;1045:357-376. doi: 10.1007/978-981-10-7230-7_16. Adv Exp Med Biol. 2018. PMID: 29896675 Review.
-
Initiation of angiogenic Kaposi's sarcoma lesions.Cancer Cell. 2003 Jan;3(1):1-3. doi: 10.1016/s1535-6108(03)00002-3. Cancer Cell. 2003. PMID: 12559168 Review.
Cited by
-
A Temporal Proteomic Map of Epstein-Barr Virus Lytic Replication in B Cells.Cell Rep. 2017 May 16;19(7):1479-1493. doi: 10.1016/j.celrep.2017.04.062. Cell Rep. 2017. PMID: 28514666 Free PMC article.
-
A specific promoter-type in ribonuclease L gene is associated with phagocytic activity in pigs.J Vet Med Sci. 2021 Sep 15;83(9):1407-1415. doi: 10.1292/jvms.21-0142. Epub 2021 Jul 28. J Vet Med Sci. 2021. PMID: 34321379 Free PMC article.
-
Oncogenic Kaposi's Sarcoma-Associated Herpesvirus Upregulates Argininosuccinate Synthase 1, a Rate-Limiting Enzyme of the Citrulline-Nitric Oxide Cycle, To Activate the STAT3 Pathway and Promote Growth Transformation.J Virol. 2019 Feb 5;93(4):e01599-18. doi: 10.1128/JVI.01599-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463977 Free PMC article.
-
Dengue Virus Induces Increased Activity of the Complement Alternative Pathway in Infected Cells.J Virol. 2018 Jun 29;92(14):e00633-18. doi: 10.1128/JVI.00633-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29743365 Free PMC article.
-
TLR4-Mediated Inflammation Promotes KSHV-Induced Cellular Transformation and Tumorigenesis by Activating the STAT3 Pathway.Cancer Res. 2017 Dec 15;77(24):7094-7108. doi: 10.1158/0008-5472.CAN-17-2321. Epub 2017 Oct 19. Cancer Res. 2017. PMID: 29051178 Free PMC article.
References
-
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM (1995) Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. New Engl J Med 332: 1186–1191. - PubMed
-
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. (1994) Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266: 1865–1869. - PubMed
-
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, et al. (1995) Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86: 1276–1280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA132637/CA/NCI NIH HHS/United States
- R01 CA096512/CA/NCI NIH HHS/United States
- R01 CA124332/CA/NCI NIH HHS/United States
- AI073099/AI/NIAID NIH HHS/United States
- R01 CA082057/CA/NCI NIH HHS/United States
- CA31363/CA/NCI NIH HHS/United States
- CA096512/CA/NCI NIH HHS/United States
- P01 CA180779/CA/NCI NIH HHS/United States
- R01 AI073099/AI/NIAID NIH HHS/United States
- R01 CA031363/CA/NCI NIH HHS/United States
- CA180779/CA/NCI NIH HHS/United States
- R01 CA115284/CA/NCI NIH HHS/United States
- R01 CA177377/CA/NCI NIH HHS/United States
- CA082057/CA/NCI NIH HHS/United States
- CA115284/CA/NCI NIH HHS/United States
- AI105809/AI/NIAID NIH HHS/United States
- R01 CA132637/CA/NCI NIH HHS/United States
- CA124332/CA/NCI NIH HHS/United States
- CA177377/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

