Structure-based design of potent and selective Leishmania N-myristoyltransferase inhibitors
- PMID: 25238611
- PMCID: PMC4211304
- DOI: 10.1021/jm5011397
Structure-based design of potent and selective Leishmania N-myristoyltransferase inhibitors
Abstract
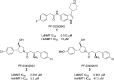
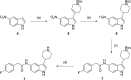
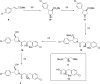
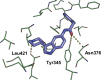
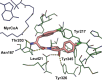
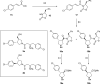
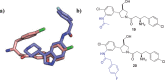
Inhibitors of Leishmania N-myristoyltransferase (NMT), a potential target for the treatment of leishmaniasis, obtained from a high-throughput screen, were resynthesized to validate activity. Crystal structures bound to Leishmania major NMT were obtained, and the active diastereoisomer of one of the inhibitors was identified. On the basis of structural insights, enzyme inhibition was increased 40-fold through hybridization of two distinct binding modes, resulting in novel, highly potent Leishmania donovani NMT inhibitors with good selectivity over the human enzyme.
Figures
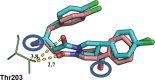
Similar articles
-
Novel Thienopyrimidine Inhibitors of Leishmania N-Myristoyltransferase with On-Target Activity in Intracellular Amastigotes.J Med Chem. 2020 Jul 23;63(14):7740-7765. doi: 10.1021/acs.jmedchem.0c00570. Epub 2020 Jul 14. J Med Chem. 2020. PMID: 32575985 Free PMC article.
-
Peptidomimetic inhibitors of N-myristoyltransferase from human malaria and leishmaniasis parasites.Org Biomol Chem. 2014 Nov 7;12(41):8132-7. doi: 10.1039/c4ob01669f. Epub 2014 Sep 18. Org Biomol Chem. 2014. PMID: 25230674 Free PMC article.
-
Development of Small-Molecule Trypanosoma brucei N-Myristoyltransferase Inhibitors: Discovery and Optimisation of a Novel Binding Mode.ChemMedChem. 2015 Nov;10(11):1821-36. doi: 10.1002/cmdc.201500301. Epub 2015 Sep 23. ChemMedChem. 2015. PMID: 26395087 Free PMC article.
-
N-Myristoyltransferase as a potential drug target in malaria and leishmaniasis.Parasitology. 2014 Jan;141(1):37-49. doi: 10.1017/S0031182013000450. Epub 2013 Apr 24. Parasitology. 2014. PMID: 23611109 Review.
-
Inhibition of protein N-myristoylation: a therapeutic protocol in developing anticancer agents.Curr Cancer Drug Targets. 2012 Jul;12(6):667-92. doi: 10.2174/156800912801784857. Curr Cancer Drug Targets. 2012. PMID: 22463587 Review.
Cited by
-
Three-dimensional structures in the design of therapeutics targeting parasitic protozoa: reflections on the past, present and future.Acta Crystallogr F Struct Biol Commun. 2015 May;71(Pt 5):485-99. doi: 10.1107/S2053230X15004987. Epub 2015 Apr 16. Acta Crystallogr F Struct Biol Commun. 2015. PMID: 25945701 Free PMC article. Review.
-
N-myristoyltransferase is a cell wall target in Aspergillus fumigatus.ACS Chem Biol. 2015 Jun 19;10(6):1425-34. doi: 10.1021/cb5008647. Epub 2015 Feb 27. ACS Chem Biol. 2015. PMID: 25706802 Free PMC article.
-
Pharmacological Validation of N-Myristoyltransferase as a Drug Target in Leishmania donovani.ACS Infect Dis. 2019 Jan 11;5(1):111-122. doi: 10.1021/acsinfecdis.8b00226. Epub 2018 Nov 12. ACS Infect Dis. 2019. PMID: 30380837 Free PMC article.
-
Drug discovery in leishmaniasis using protein lipidation as a target.Biophys Rev. 2021 Nov 4;13(6):1139-1146. doi: 10.1007/s12551-021-00855-0. eCollection 2021 Dec. Biophys Rev. 2021. PMID: 35035594 Free PMC article. Review.
-
Structure and Functional Diversity of GCN5-Related N-Acetyltransferases (GNAT).Int J Mol Sci. 2016 Jun 28;17(7):1018. doi: 10.3390/ijms17071018. Int J Mol Sci. 2016. PMID: 27367672 Free PMC article. Review.
References
-
- Leishmaniasis: Burden and Distribution; World Health Organization: Geneva, 2013; http://www.who.int/leishmaniasis/burden/en (accessed July 1, 2014).
-
- den Boer M.; Argaw D.; Jannin J.; Alvar J. Leishmaniasis impact and treatment access. Clin. Microbiol. Infect. 2011, 17, 1471–1477. - PubMed
-
- Sundar S. Drug resistance in Indian visceral leishmaniasis. Trop. Med. Int. Health 2001, 6, 849–854. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

