Effects of tumor microenvironment heterogeneity on nanoparticle disposition and efficacy in breast cancer tumor models
- PMID: 25231403
- PMCID: PMC4565518
- DOI: 10.1158/1078-0432.CCR-14-0493
Effects of tumor microenvironment heterogeneity on nanoparticle disposition and efficacy in breast cancer tumor models
Abstract
Purpose: Tumor cells are surrounded by a complex microenvironment. The purpose of our study was to evaluate the role of heterogeneity of the tumor microenvironment in the variability of nanoparticle (NP) delivery and efficacy.
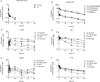
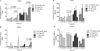
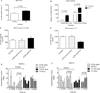
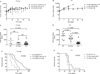
Experimental designs: C3(1)-T-Antigen genetically engineered mouse model (C3-TAg) and T11/TP53(Null) orthotopic syngeneic murine transplant model (T11) representing human breast tumor subtypes basal-like and claudin-low, respectively, were evaluated. For the pharmacokinetic studies, non-liposomal doxorubicin (NL-doxo) or polyethylene glycol tagged (PEGylated) liposomal doxorubicin (PLD) was administered at 6 mg/kg i.v. x1. Area under the concentration versus time curve (AUC) of doxorubicin was calculated. Macrophages, collagen, and the amount of vasculature were assessed by IHC. Chemokines and cytokines were measured by multiplex immunochemistry. NL-doxo or PLD was administered at 6 mg/kg i.v. weekly x6 in efficacy studies. Analyses of intermediary tumor response and overall survival were performed.
Results: Plasma AUC of NL-doxo and PLD encapsulated and released doxorubicin was similar between two models. However, tumor sum total AUC of PLD was 2-fold greater in C3-TAg compared with T11 (P < 0.05). T11 tumors showed significantly higher expression of CC chemokine ligand (CCL) 2 and VEGF-a, greater vascular quantity, and decreased expression of VEGF-c compared with C3-TAg (P < 0.05). PLD was more efficacious compared with NL-doxo in both models.
Conclusion: The tumor microenvironment and/or tumor cell features of breast cancer affected NP tumor delivery and efficacy, but not the small-molecule drug. Our findings reveal the role of the tumor microenvironment in variability of NP delivery and therapeutic outcomes.
©2014 American Association for Cancer Research.
Conflict of interest statement
Figures


Similar articles
-
Pharmacokinetics and efficacy of PEGylated liposomal doxorubicin in an intracranial model of breast cancer.PLoS One. 2013 May 1;8(5):e61359. doi: 10.1371/journal.pone.0061359. Print 2013. PLoS One. 2013. PMID: 23650496 Free PMC article.
-
Roles of chemokines CCL2 and CCL5 in the pharmacokinetics of PEGylated liposomal doxorubicin in vivo and in patients with recurrent epithelial ovarian cancer.Nanomedicine. 2015 Oct;11(7):1797-807. doi: 10.1016/j.nano.2015.05.007. Epub 2015 Jun 17. Nanomedicine. 2015. PMID: 26093057
-
Improved drug delivery and therapeutic efficacy of PEgylated liposomal doxorubicin by targeting anti-HER2 peptide in murine breast tumor model.Eur J Pharm Sci. 2016 Apr 30;86:125-35. doi: 10.1016/j.ejps.2016.03.009. Epub 2016 Mar 10. Eur J Pharm Sci. 2016. PMID: 26972276
-
New insights and evolving role of pegylated liposomal doxorubicin in cancer therapy.Drug Resist Updat. 2016 Nov;29:90-106. doi: 10.1016/j.drup.2016.10.003. Epub 2016 Oct 29. Drug Resist Updat. 2016. PMID: 27912846 Review.
-
The role of chemokines in breast cancer pathology and its possible use as therapeutic targets.J Immunol Res. 2014;2014:849720. doi: 10.1155/2014/849720. Epub 2014 Aug 5. J Immunol Res. 2014. PMID: 25165728 Free PMC article. Review.
Cited by
-
Multimodal imaging of metabolic activities for distinguishing subtypes of breast cancer.Biomed Opt Express. 2023 Oct 13;14(11):5764-5780. doi: 10.1364/BOE.500252. eCollection 2023 Nov 1. Biomed Opt Express. 2023. PMID: 38021123 Free PMC article.
-
Synchronous Intravital Imaging and Cavitation Monitoring of Antivascular Focused Ultrasound in Tumor Microvasculature Using Monodisperse Low Boiling Point Nanodroplets.ACS Nano. 2024 Jan 9;18(1):410-427. doi: 10.1021/acsnano.3c07711. Epub 2023 Dec 26. ACS Nano. 2024. PMID: 38147452 Free PMC article.
-
Deep Tumor Penetration of Doxorubicin-Loaded Glycol Chitosan Nanoparticles Using High-Intensity Focused Ultrasound.Pharmaceutics. 2020 Oct 15;12(10):974. doi: 10.3390/pharmaceutics12100974. Pharmaceutics. 2020. PMID: 33076520 Free PMC article.
-
Revisiting the outstanding questions in cancer nanomedicine with a future outlook.Nanoscale Adv. 2021 Dec 22;4(3):634-653. doi: 10.1039/d1na00810b. eCollection 2022 Feb 1. Nanoscale Adv. 2021. PMID: 36131837 Free PMC article. Review.
-
A nanoparticle-incorporated STING activator enhances antitumor immunity in PD-L1-insensitive models of triple-negative breast cancer.JCI Insight. 2018 Nov 15;3(22):e120638. doi: 10.1172/jci.insight.120638. JCI Insight. 2018. PMID: 30429378 Free PMC article.
References
-
- Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS nano. 2009;3(1):16–20. - PubMed
-
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature nanotechnology. 2007;2(12):751–60. - PubMed
-
- Maeda H, Greish K, Fang J. Polymer Therapeutics II. Springer; 2006. The EPR effect and polymeric drugs: A paradigm shift for cancer chemotherapy in the 21st century; pp. 103–221.
-
- Zamboni WC. Concept and clinical evaluation of carrier-mediated anticancer agents. Oncologist. 2008;13(3):248–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

