Dopamine D2 receptor agonists inhibit lung cancer progression by reducing angiogenesis and tumor infiltrating myeloid derived suppressor cells
- PMID: 25226814
- PMCID: PMC4277897
- DOI: 10.1016/j.molonc.2014.08.008
Dopamine D2 receptor agonists inhibit lung cancer progression by reducing angiogenesis and tumor infiltrating myeloid derived suppressor cells
Abstract
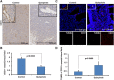
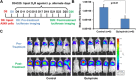
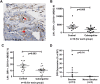
We sought to determine whether Dopamine D2 Receptor (D2R) agonists inhibit lung tumor progression and identify subpopulations of lung cancer patients that benefit most from D2R agonist therapy. We demonstrate D2R agonists abrogate lung tumor progression in syngeneic (LLC1) and human xenograft (A549) orthotopic murine models through inhibition of tumor angiogenesis and reduction of tumor infiltrating myeloid derived suppressor cells. Pathological examination of human lung cancer tissue revealed a positive correlation between endothelial D2R expression and tumor stage. Lung cancer patients with a smoking history exhibited greater levels of D2R in lung endothelium. Our results suggest D2R agonists may represent a promising individualized therapy for lung cancer patients with high levels of endothelial D2R expression and a smoking history.
Keywords: Angiogenesis; Cabergoline; Dopamine; Dopamine D2 receptor agonists; Lung cancer; VEGF.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Allosteric interactions between agonists and antagonists within the adenosine A2A receptor-dopamine D2 receptor heterotetramer.Proc Natl Acad Sci U S A. 2015 Jul 7;112(27):E3609-18. doi: 10.1073/pnas.1507704112. Epub 2015 Jun 22. Proc Natl Acad Sci U S A. 2015. PMID: 26100888 Free PMC article.
-
The Dopamine D2 Receptor Contributes to the Spheroid Formation Behavior of U87 Glioblastoma Cells.Pharmacology. 2020;105(1-2):19-27. doi: 10.1159/000502562. Epub 2019 Oct 23. Pharmacology. 2020. PMID: 31645049 Free PMC article.
-
Complementary actions of dopamine D2 receptor agonist and anti-vegf therapy on tumoral vessel normalization in a transgenic mouse model.Int J Cancer. 2017 May 1;140(9):2150-2161. doi: 10.1002/ijc.30628. Epub 2017 Feb 14. Int J Cancer. 2017. PMID: 28152577
-
Multivalent approaches and beyond: novel tools for the investigation of dopamine D2 receptor pharmacology.Future Med Chem. 2016 Jul;8(11):1349-72. doi: 10.4155/fmc-2016-0010. Epub 2016 Jun 30. Future Med Chem. 2016. PMID: 27357619 Review.
-
Allosteric mechanisms within the adenosine A2A-dopamine D2 receptor heterotetramer.Neuropharmacology. 2016 May;104:154-60. doi: 10.1016/j.neuropharm.2015.05.028. Epub 2015 Jun 4. Neuropharmacology. 2016. PMID: 26051403 Free PMC article. Review.
Cited by
-
Therapeutic strategies for colorectal cancer: antitumor efficacy of dopamine D2 receptor antagonists.Toxicol Res. 2024 Aug 7;40(4):533-540. doi: 10.1007/s43188-024-00259-8. eCollection 2024 Oct. Toxicol Res. 2024. PMID: 39345737 Review.
-
The role of the ALKBH5 RNA demethylase in invasive breast cancer.Discov Oncol. 2024 Aug 11;15(1):343. doi: 10.1007/s12672-024-01205-8. Discov Oncol. 2024. PMID: 39127986 Free PMC article.
-
DRD2 activation inhibits choroidal neovascularization in patients with Parkinson's disease and age-related macular degeneration.J Clin Invest. 2024 Jul 16;134(17):e174199. doi: 10.1172/JCI174199. J Clin Invest. 2024. PMID: 39012703 Free PMC article.
-
Involvement of neuronal factors in tumor angiogenesis and the shaping of the cancer microenvironment.Front Immunol. 2024 Feb 5;15:1284629. doi: 10.3389/fimmu.2024.1284629. eCollection 2024. Front Immunol. 2024. PMID: 38375479 Free PMC article. Review.
-
The cancer-immune dialogue in the context of stress.Nat Rev Immunol. 2024 Apr;24(4):264-281. doi: 10.1038/s41577-023-00949-8. Epub 2023 Oct 13. Nat Rev Immunol. 2024. PMID: 37833492 Review.
References
-
- Alberg, A.J. , Shopland, D.R. , Cummings, K.M. , 2014. The 2014 Surgeon General's report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am. J. Epidemiol. 179, 403–412. - PMC - PubMed
-
- Basu, S. , Nagy, J.A. , Pal, S. , Vasile, E. , Eckelhoefer, I.A. , Bliss, V.S. , Manseau, E.J. , Dasgupta, P.S. , Dvorak, H.F. , Mukhopadhyay, D. , 2001. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat. Med. 7, 569–574. - PubMed
-
- Basu, S. , Sarkar, C. , Chakroborty, D. , Nagy, J. , Mitra, R.B. , Dasgupta, P.S. , Mukhopadhyay, D. , 2004. Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis. Cancer Res. 64, 5551–5555. - PubMed
-
- Bhandarkar, S.S. , Jaconi, M. , Fried, L.E. , Bonner, M.Y. , Lefkove, B. , Govindarajan, B. , Perry, B.N. , Parhar, R. , Mackelfresh, J. , Sohn, A. , Stouffs, M. , Knaus, U. , Yancopoulos, G. , Reiss, Y. , Benest, A.V. , Augustin, H.G. , Arbiser, J.L. , 2009. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J. Clin. Invest. 119, 2359–2365. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

