An independent study of the preclinical efficacy of C2-8 in the R6/2 transgenic mouse model of Huntington's disease
- PMID: 25062731
- PMCID: PMC4124458
- DOI: 10.3233/JHD-130074
An independent study of the preclinical efficacy of C2-8 in the R6/2 transgenic mouse model of Huntington's disease
Abstract
Background: C2-8 is a small molecule inhibitor of polyglutamine aggregation and can reduce photoreceptor neurodegeneration in a Drosophila model of Huntington's disease (HD). Further preclinical studies have shown that oral administration of C2-8 in R6/2 HD transgenic mice can penetrate into the brain, reduce mHTT-exon1 aggregation, improve motor performance and diminish striatal neuron atrophy.
Objective: In this independent preclinical study, we aimed to evaluate the pharmacokinetic properties and therapeutic efficacy of C2-8 intraperitoneal (IP) delivery in the R6/2 HD mouse.
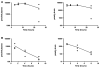
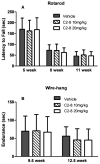
Methods: R6/2 mice were IP injected with low dose C2-8 (10 mg/kg), high dose C2-8 (20 mg/kg), or vehicle twice daily from 3 weeks to 3 months old. Longitudinal behavioral tests (accelerating Rotarod and wire-hang) were performed to evaluate the motor deficits, and neuropathology was measured by unbiased stereology.
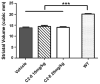
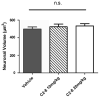
Results: We confirmed that the compound has good blood-brain-barrier penetration after acute or sub-chronic intraperitoneal delivery. Chronic treatment with C2-8 in R6/2 mice results in a significant reduction of nuclear mHTT aggregate volume in the brains, replicating a key finding of C2-8 as a polyglutamine aggregation inhibitor in vivo. However, by comparing HD mice with C2-8 treatment to those with vehicle treatment, we were unable to demonstrate significant amelioration of motor deficits using Rotarod and wire-hang tests. Moreover, we did not observe improvement in the striatal neurodegenerative pathology, as measured by brain weight, striatal volume, and striatal neuron volume in the C2-8 treated R6/2 mice.
Conclusions: Our study supports the practice of independent preclinical studies for novel molecules in HD therapeutic development and suggests that the use of alternative delivery strategies and full-length HD mouse models are likely needed to further assess whether the aggregate-inhibiting properties of C2-8 can be consistently translated into a preclinical benefit in HD mice.
Keywords: C2-8; Huntington's disease; R6/2; aggregate; huntingtin; preclinical.
Conflict of interest statement
Figures

Similar articles
-
A small-molecule therapeutic lead for Huntington's disease: preclinical pharmacology and efficacy of C2-8 in the R6/2 transgenic mouse.Proc Natl Acad Sci U S A. 2007 Oct 16;104(42):16685-9. doi: 10.1073/pnas.0707842104. Epub 2007 Oct 9. Proc Natl Acad Sci U S A. 2007. PMID: 17925440 Free PMC article.
-
Treatment with a herbal formula B401 enhances neuroprotection and angiogenesis in the R6/2 mouse model of Huntington's disease.Drug Des Devel Ther. 2015 Feb 16;9:887-900. doi: 10.2147/DDDT.S78015. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25733809 Free PMC article.
-
The antidepressant sertraline improves the phenotype, promotes neurogenesis and increases BDNF levels in the R6/2 Huntington's disease mouse model.Exp Neurol. 2008 Mar;210(1):154-63. doi: 10.1016/j.expneurol.2007.10.015. Epub 2007 Nov 9. Exp Neurol. 2008. PMID: 18096160 Free PMC article.
-
The R6 lines of transgenic mice: a model for screening new therapies for Huntington's disease.Brain Res Rev. 2009 Mar;59(2):410-31. doi: 10.1016/j.brainresrev.2008.12.001. Epub 2008 Dec 16. Brain Res Rev. 2009. PMID: 19118572 Review.
-
Murine Models of Huntington's Disease for Evaluating Therapeutics.Methods Mol Biol. 2018;1780:179-207. doi: 10.1007/978-1-4939-7825-0_10. Methods Mol Biol. 2018. PMID: 29856020 Review.
Cited by
-
Studying Huntington's Disease in Yeast: From Mechanisms to Pharmacological Approaches.Front Mol Neurosci. 2018 Sep 4;11:318. doi: 10.3389/fnmol.2018.00318. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30233317 Free PMC article. Review.
-
Protein Aggregation Inhibitors as Disease-Modifying Therapies for Polyglutamine Diseases.Front Neurosci. 2021 Feb 12;15:621996. doi: 10.3389/fnins.2021.621996. eCollection 2021. Front Neurosci. 2021. PMID: 33642983 Free PMC article. Review.
-
Yeast Models for Amyloids and Prions: Environmental Modulation and Drug Discovery.Molecules. 2019 Sep 18;24(18):3388. doi: 10.3390/molecules24183388. Molecules. 2019. PMID: 31540362 Free PMC article. Review.
-
Therapeutic Advances for Huntington's Disease.Brain Sci. 2020 Jan 12;10(1):43. doi: 10.3390/brainsci10010043. Brain Sci. 2020. PMID: 31940909 Free PMC article. Review.
-
Neurodegenerative Diseases: New Hopes and Perspectives.Curr Mol Med. 2024;24(8):1004-1032. doi: 10.2174/1566524023666230907093451. Curr Mol Med. 2024. PMID: 37691199 Review.
References
-
- Zhang X, Smith DL, Meriin AB, Engemann S, Russel DE, Roark M, Washington SL, Maxwell MM, Marsh JL, Thompson LM, Wanker EE, Young AB, Housman DE, Bates GP, Sherman MY, Kazantsev AG. A potent small molecule inhibits polyglutamine aggregation in Huntington's disease neurons and suppresses neurodegeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:892–7. - PMC - PubMed
-
- Chopra V, Fox JH, Lieberman G, Dorsey K, Matson W, Waldmeier P, Housman DE, Kazantsev A, Young AB, Hersch S. A small-molecule therapeutic lead for Huntington's disease: Preclinical pharmacology and efficacy of C2-8 in the R6/2 transgenic mouse. Proc Natl Acad Sci U S A. 2007;104:16685–9. - PMC - PubMed
-
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. - PubMed
-
- Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT, 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–91. - PMC - PubMed
-
- Gray M, Shirasaki DI, Cepeda C, André VM, Wilburn B, Lu XH, Tao J, Yamazaki I, Li SH, Sun YE, Li XJ, Levine MS, Yang XW. Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J Neurosci. 2008;28:6182–95. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

