Sialyllactose ameliorates myopathic phenotypes in symptomatic GNE myopathy model mice
- PMID: 25062695
- PMCID: PMC4172045
- DOI: 10.1093/brain/awu210
Sialyllactose ameliorates myopathic phenotypes in symptomatic GNE myopathy model mice
Abstract
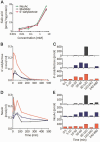
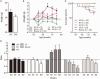
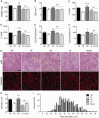
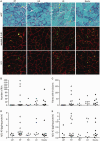
Patients with GNE myopathy, a progressive and debilitating disease caused by a genetic defect in sialic acid biosynthesis, rely on supportive care and eventually become wheelchair-bound. To elucidate whether GNE myopathy is treatable at a progressive stage of the disease, we examined the efficacy of sialic acid supplementation on symptomatic old GNE myopathy mice that have ongoing, active muscle degeneration. We examined the therapeutic effect of a less metabolized sialic acid compound (6'-sialyllactose) or free sialic acid (N-acetylneuraminic acid) by oral, continuous administration to 50-week-old GNE myopathy mice for 30 weeks. To evaluate effects on their motor performance in living mice, spontaneous locomotion activity on a running wheel was measured chronologically at 50, 65, 72 and 80 weeks of age. The size, force production, and pathology of isolated gastrocnemius muscle were analysed at the end point. Sialic acid level in skeletal muscle was also measured. Spontaneous locomotion activity was recovered in 6'-sialyllactose-treated mice, while NeuAc-treated mice slowed the disease progression. Treatment with 6'-sialyllactose led to marked restoration of hyposialylation in muscle and consequently to robust improvement in the muscle size, contractile parameters, and pathology as compared to NeuAc. This is due to the fact that 6'-sialyllactose is longer working as it is further metabolized to free sialic acid after initial absorption. 6'-sialyllactose ameliorated muscle atrophy and degeneration in symptomatic GNE myopathy mice. Our results provide evidence that GNE myopathy can be treated even at a progressive stage and 6'-sialyllactose has more remarkable advantage than free sialic acid, providing a conceptual proof for clinical use in patients.
Keywords: GNE myopathy; amyloid inclusion; distal myopathy with rimmed vacuoles (DMRV)/hereditary inclusion body myopathy (hIBM); hyposialylation; sialyllactose.
© The Author (2014). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
[Animal model of distal myopathy with rimmed vacuoles/hereditary inclusion body myopathy and preclinical trial with sugar compounds].Brain Nerve. 2010 Jun;62(6):601-7. Brain Nerve. 2010. PMID: 20548120 Review. Japanese.
-
A Gne knockout mouse expressing human GNE D176V mutation develops features similar to distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy.Hum Mol Genet. 2007 Nov 15;16(22):2669-82. doi: 10.1093/hmg/ddm220. Epub 2007 Aug 18. Hum Mol Genet. 2007. PMID: 17704511
-
Peracetylated N-acetylmannosamine, a synthetic sugar molecule, efficiently rescues muscle phenotype and biochemical defects in mouse model of sialic acid-deficient myopathy.J Biol Chem. 2012 Jan 20;287(4):2689-705. doi: 10.1074/jbc.M111.297051. Epub 2011 Dec 8. J Biol Chem. 2012. PMID: 22157763 Free PMC article.
-
[Development of therapy for distal myopathy with rimmed vacuoles].Rinsho Shinkeigaku. 2009 Nov;49(11):852-5. doi: 10.5692/clinicalneurol.49.852. Rinsho Shinkeigaku. 2009. PMID: 20030229 Review. Japanese.
-
Pharmacokinetics and clinical efficacy of 6'-sialyllactose in patients with GNE myopathy: Randomized pilot trial.Biomed Pharmacother. 2023 Dec;168:115689. doi: 10.1016/j.biopha.2023.115689. Epub 2023 Oct 16. Biomed Pharmacother. 2023. PMID: 37852099 Clinical Trial.
Cited by
-
6-sialyllactose ameliorates dihydrotestosterone-induced benign prostatic hyperplasia through suppressing VEGF-mediated angiogenesis.BMB Rep. 2019 Sep;52(9):560-565. doi: 10.5483/BMBRep.2019.52.9.113. BMB Rep. 2019. PMID: 31383249 Free PMC article.
-
Pharmacological activation of SERCA ameliorates dystrophic phenotypes in dystrophin-deficient mdx mice.Hum Mol Genet. 2021 May 31;30(11):1006-1019. doi: 10.1093/hmg/ddab100. Hum Mol Genet. 2021. PMID: 33822956 Free PMC article.
-
Muscle Weakness and Fibrosis Due to Cell Autonomous and Non-cell Autonomous Events in Collagen VI Deficient Congenital Muscular Dystrophy.EBioMedicine. 2017 Feb;15:193-202. doi: 10.1016/j.ebiom.2016.12.011. Epub 2016 Dec 23. EBioMedicine. 2017. PMID: 28043812 Free PMC article.
-
Quantitative hydrophilic interaction chromatography-mass spectrometry analysis of N-acetylneuraminic acid and N-acetylmannosamine in human plasma.J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Sep 1;1000:105-11. doi: 10.1016/j.jchromb.2015.07.018. Epub 2015 Jul 17. J Chromatogr B Analyt Technol Biomed Life Sci. 2015. PMID: 26218770 Free PMC article.
-
GNE Myopathy: Etiology, Diagnosis, and Therapeutic Challenges.Neurotherapeutics. 2018 Oct;15(4):900-914. doi: 10.1007/s13311-018-0671-y. Neurotherapeutics. 2018. PMID: 30338442 Free PMC article. Review.
References
-
- ClinicalTrials.gov [Internet] A service of the U.S. National Institute of Health. Available from: http://www.clinicaltrials.gov./ (18 April 2014, date last accessed)
-
- Drouillard S, Mine T, Kajiwara H, Yamamoto T, Samain E. Efficient synthesis of 6’-sialyllactose, 6,6’-disialyllactose, and 6’-KDO-lactose by metabolically engineered E. coli expressing a multifunctional sialyltransferase from the Photobacterium sp. JT-ISH-224. Carbohydr Res. 2010;345:1394–9. - PubMed
-
- Eisenberg I, Avidan N, Potikha T, Hochner H, Chen M, Olender T, et al. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet. 2001;29:83–7. - PubMed
-
- Endo S, Morita M, Ueno M, Maeda T, Terabayashi T. Fluorescent labeling of a carboxyl group of sialic acid for MALDI-MS analysis of sialyloligosaccharides and ganglioside. Biochem Biophys Res Commun. 2009;378:890–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

