Lysosome: regulator of lipid degradation pathways
- PMID: 25061009
- PMCID: PMC4247383
- DOI: 10.1016/j.tcb.2014.06.006
Lysosome: regulator of lipid degradation pathways
Abstract
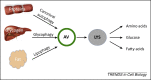
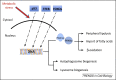
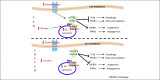
Autophagy is a catabolic pathway that has a fundamental role in the adaptation to fasting and primarily relies on the activity of the endolysosomal system, to which the autophagosome targets substrates for degradation. Recent studies have revealed that the lysosomal-autophagic pathway plays an important part in the early steps of lipid degradation. In this review, we discuss the transcriptional mechanisms underlying co-regulation between lysosome, autophagy, and other steps of lipid catabolism, including the activity of nutrient-sensitive transcription factors (TFs) and of members of the nuclear receptor family. In addition, we discuss how the lysosome acts as a metabolic sensor and orchestrates the transcriptional response to fasting.
Keywords: Autophagy; FOXOs; TFEB; TP53; lipophagy; lysosome; mTORC1; nuclear receptors; transcription factors.
Copyright © 2014 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
Similar articles
-
Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion.Cell Res. 2013 Apr;23(4):508-23. doi: 10.1038/cr.2013.11. Epub 2013 Jan 22. Cell Res. 2013. PMID: 23337583 Free PMC article.
-
At the end of the autophagic road: an emerging understanding of lysosomal functions in autophagy.Trends Biochem Sci. 2014 Feb;39(2):61-71. doi: 10.1016/j.tibs.2013.12.001. Epub 2013 Dec 24. Trends Biochem Sci. 2014. PMID: 24369758 Review.
-
Endocytosis and autophagy: Shared machinery for degradation.Bioessays. 2013 Jan;35(1):34-45. doi: 10.1002/bies.201200130. Epub 2012 Nov 13. Bioessays. 2013. PMID: 23147242 Review.
-
Dual suppressive effect of MTORC1 on autophagy: tame the dragon by shackling both the head and the tail.Autophagy. 2013 May;9(5):803-5. doi: 10.4161/auto.23965. Epub 2013 Feb 25. Autophagy. 2013. PMID: 23439250 Free PMC article.
-
Autophagy, lipophagy and lysosomal lipid storage disorders.Biochim Biophys Acta. 2016 Apr;1861(4):269-84. doi: 10.1016/j.bbalip.2016.01.006. Epub 2016 Jan 14. Biochim Biophys Acta. 2016. PMID: 26778751 Review.
Cited by
-
Targeted protein degradation: advances in drug discovery and clinical practice.Signal Transduct Target Ther. 2024 Nov 6;9(1):308. doi: 10.1038/s41392-024-02004-x. Signal Transduct Target Ther. 2024. PMID: 39500878 Free PMC article. Review.
-
TFEB controls sensitivity to chemotherapy and immuno-killing in non-small cell lung cancer.J Exp Clin Cancer Res. 2024 Aug 7;43(1):219. doi: 10.1186/s13046-024-03142-4. J Exp Clin Cancer Res. 2024. PMID: 39107857 Free PMC article.
-
mTORC1 Signaling in Brain Endothelial Progenitors Contributes to CCM Pathogenesis.Circ Res. 2024 Aug 2;135(4):e94-e113. doi: 10.1161/CIRCRESAHA.123.324015. Epub 2024 Jul 3. Circ Res. 2024. PMID: 38957991
-
Role of TFEB in Huntington's Disease.Biology (Basel). 2024 Apr 4;13(4):238. doi: 10.3390/biology13040238. Biology (Basel). 2024. PMID: 38666850 Free PMC article. Review.
-
Glycerophosphodiesters inhibit lysosomal phospholipid catabolism in Batten disease.Mol Cell. 2024 Apr 4;84(7):1354-1364.e9. doi: 10.1016/j.molcel.2024.02.006. Epub 2024 Mar 5. Mol Cell. 2024. PMID: 38447580
References
-
- Wang T. The comparative physiology of food deprivation: from feast to famine. Annu. Rev. Physiol. 2006;68:223–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

