Structural insights into the human metapneumovirus glycoprotein ectodomain
- PMID: 25031352
- PMCID: PMC4178817
- DOI: 10.1128/JVI.01726-14
Structural insights into the human metapneumovirus glycoprotein ectodomain
Abstract
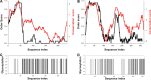
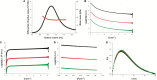
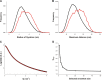
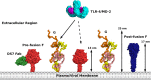
Human metapneumovirus is a major cause of respiratory tract infections worldwide. Previous reports have shown that the viral attachment glycoprotein (G) modulates innate and adaptive immune responses, leading to incomplete immunity and promoting reinfection. Using bioinformatics analyses, static light scattering, and small-angle X-ray scattering, we show that the extracellular region of G behaves as a heavily glycosylated, intrinsically disordered polymer. We discuss potential implications of these findings for the modulation of immune responses by G.
Copyright © 2014 Leyrat et al.
Figures
Similar articles
-
Structural dissection of human metapneumovirus phosphoprotein using small angle x-ray scattering.Sci Rep. 2017 Nov 1;7(1):14865. doi: 10.1038/s41598-017-14448-z. Sci Rep. 2017. PMID: 29093501 Free PMC article.
-
Human metapneumovirus glycoprotein G disrupts mitochondrial signaling in airway epithelial cells.PLoS One. 2013 Apr 23;8(4):e62568. doi: 10.1371/journal.pone.0062568. Print 2013. PLoS One. 2013. PMID: 23626834 Free PMC article.
-
Individual contributions of the human metapneumovirus F, G, and SH surface glycoproteins to the induction of neutralizing antibodies and protective immunity.Virology. 2006 Feb 20;345(2):492-501. doi: 10.1016/j.virol.2005.10.016. Epub 2005 Nov 21. Virology. 2006. PMID: 16300813
-
Human Metapneumovirus: Mechanisms and Molecular Targets Used by the Virus to Avoid the Immune System.Front Immunol. 2018 Oct 24;9:2466. doi: 10.3389/fimmu.2018.02466. eCollection 2018. Front Immunol. 2018. PMID: 30405642 Free PMC article. Review.
-
Modulation of Host Immunity by Human Respiratory Syncytial Virus Virulence Factors: A Synergic Inhibition of Both Innate and Adaptive Immunity.Front Cell Infect Microbiol. 2017 Aug 16;7:367. doi: 10.3389/fcimb.2017.00367. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28861397 Free PMC article. Review.
Cited by
-
Molecular characterization of human respiratory syncytial virus in Seoul, South Korea, during 10 consecutive years, 2010-2019.PLoS One. 2023 Apr 6;18(4):e0283873. doi: 10.1371/journal.pone.0283873. eCollection 2023. PLoS One. 2023. PMID: 37023101 Free PMC article.
-
Human metapneumovirus small hydrophobic (SH) protein downregulates type I IFN pathway signaling by affecting STAT1 expression and phosphorylation.Virology. 2016 Jul;494:248-56. doi: 10.1016/j.virol.2016.04.022. Epub 2016 Apr 27. Virology. 2016. PMID: 27131212 Free PMC article.
-
Structural basis for recognition of the central conserved region of RSV G by neutralizing human antibodies.PLoS Pathog. 2018 Mar 6;14(3):e1006935. doi: 10.1371/journal.ppat.1006935. eCollection 2018 Mar. PLoS Pathog. 2018. PMID: 29509814 Free PMC article.
-
Timing is everything: Fine-tuned molecular machines orchestrate paramyxovirus entry.Virology. 2015 May;479-480:518-31. doi: 10.1016/j.virol.2015.02.037. Epub 2015 Mar 12. Virology. 2015. PMID: 25771804 Free PMC article. Review.
-
Structure of the N-RNA/P interface indicates mode of L/P recruitment to the nucleocapsid of human metapneumovirus.Nat Commun. 2023 Nov 22;14(1):7627. doi: 10.1038/s41467-023-43434-5. Nat Commun. 2023. PMID: 37993464 Free PMC article.
References
-
- Boivin G, Abed Y, Pelletier G, Ruel L, Moisan D, Cote S, Peret TC, Erdman DD, Anderson LJ. 2002. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J. Infect. Dis. 186:1330–1334. 10.1086/344319 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

