Aldehyde tag coupled with HIPS chemistry enables the production of ADCs conjugated site-specifically to different antibody regions with distinct in vivo efficacy and PK outcomes
- PMID: 24924618
- PMCID: PMC4215875
- DOI: 10.1021/bc500189z
Aldehyde tag coupled with HIPS chemistry enables the production of ADCs conjugated site-specifically to different antibody regions with distinct in vivo efficacy and PK outcomes
Abstract
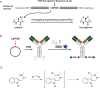
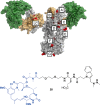
It is becoming increasingly clear that site-specific conjugation offers significant advantages over conventional conjugation chemistries used to make antibody-drug conjugates (ADCs). Site-specific payload placement allows for control over both the drug-to-antibody ratio (DAR) and the conjugation site, both of which play an important role in governing the pharmacokinetics (PK), disposition, and efficacy of the ADC. In addition to the DAR and site of conjugation, linker composition also plays an important role in the properties of an ADC. We have previously reported a novel site-specific conjugation platform comprising linker payloads designed to selectively react with site-specifically engineered aldehyde tags on an antibody backbone. This chemistry results in a stable C-C bond between the antibody and the cytotoxin payload, providing a uniquely stable connection with respect to the other linker chemistries used to generate ADCs. The flexibility and versatility of the aldehyde tag conjugation platform has enabled us to undertake a systematic evaluation of the impact of conjugation site and linker composition on ADC properties. Here, we describe the production and characterization of a panel of ADCs bearing the aldehyde tag at different locations on an IgG1 backbone conjugated using Hydrazino-iso-Pictet-Spengler (HIPS) chemistry. We demonstrate that in a panel of ADCs with aldehyde tags at different locations, the site of conjugation has a dramatic impact on in vivo efficacy and pharmacokinetic behavior in rodents; this advantage translates to an improved safety profile in rats as compared to a conventional lysine conjugate.
Figures
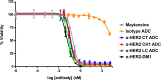
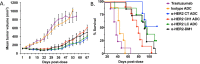
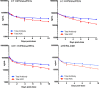
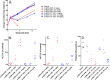
Similar articles
-
Antibody-Drug Conjugates (ADCs) Derived from Interchain Cysteine Cross-Linking Demonstrate Improved Homogeneity and Other Pharmacological Properties over Conventional Heterogeneous ADCs.Mol Pharm. 2015 Nov 2;12(11):3986-98. doi: 10.1021/acs.molpharmaceut.5b00432. Epub 2015 Oct 2. Mol Pharm. 2015. PMID: 26393951 Free PMC article.
-
In vitro and in vivo evaluation of cysteine and site specific conjugated herceptin antibody-drug conjugates.PLoS One. 2014 Jan 14;9(1):e83865. doi: 10.1371/journal.pone.0083865. eCollection 2014. PLoS One. 2014. PMID: 24454709 Free PMC article.
-
Exploring the effects of linker composition on site-specifically modified antibody-drug conjugates.Eur J Med Chem. 2014 Dec 17;88:3-9. doi: 10.1016/j.ejmech.2014.08.062. Epub 2014 Aug 23. Eur J Med Chem. 2014. PMID: 25176286
-
Antibody-drug conjugates: recent advances in conjugation and linker chemistries.Protein Cell. 2018 Jan;9(1):33-46. doi: 10.1007/s13238-016-0323-0. Epub 2016 Oct 14. Protein Cell. 2018. PMID: 27743348 Free PMC article. Review.
-
Current methods for the synthesis of homogeneous antibody-drug conjugates.Biotechnol Adv. 2015 Nov 1;33(6 Pt 1):775-84. doi: 10.1016/j.biotechadv.2015.05.001. Epub 2015 May 14. Biotechnol Adv. 2015. PMID: 25981886 Review.
Cited by
-
Antibody-Drug Conjugates: Functional Principles and Applications in Oncology and Beyond.Vaccines (Basel). 2021 Sep 29;9(10):1111. doi: 10.3390/vaccines9101111. Vaccines (Basel). 2021. PMID: 34696218 Free PMC article. Review.
-
Site-specifically labeled 89Zr-DFO-trastuzumab improves immuno-reactivity and tumor uptake for immuno-PET in a subcutaneous HER2-positive xenograft mouse model.Theranostics. 2019 Jun 9;9(15):4409-4420. doi: 10.7150/thno.32883. eCollection 2019. Theranostics. 2019. PMID: 31285769 Free PMC article.
-
Progress and Challenges in Developing Aptamer-Functionalized Targeted Drug Delivery Systems.Int J Mol Sci. 2015 Oct 9;16(10):23784-822. doi: 10.3390/ijms161023784. Int J Mol Sci. 2015. PMID: 26473828 Free PMC article. Review.
-
Formylglycine, a post-translationally generated residue with unique catalytic capabilities and biotechnology applications.ACS Chem Biol. 2015 Jan 16;10(1):72-84. doi: 10.1021/cb500897w. ACS Chem Biol. 2015. PMID: 25514000 Free PMC article. Review.
-
Recent Developments in ADC Technology: Preclinical Studies Signal Future Clinical Trends.BioDrugs. 2017 Dec;31(6):521-531. doi: 10.1007/s40259-017-0254-1. BioDrugs. 2017. PMID: 29119409 Free PMC article. Review.
References
-
- Sievers E. L.; Senter P. D. (2013) Antibody-drug conjugates in cancer therapy. Annu. Rev. Med. 64, 15–29. - PubMed
-
- Mullard A. (2013) Maturing antibody-drug conjugate pipeline hits 30. Nat. Rev. Drug Discovery 12, 329–332. - PubMed
-
- Hamblett K. J.; et al. (2004) Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res. 10, 7063–7070. - PubMed
-
- Junutula J. R.; et al. (2008) Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat. Biotechnol. 26, 925–932. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

