Kaposi's sarcoma-associated herpesvirus K3 and K5 ubiquitin E3 ligases have stage-specific immune evasion roles during lytic replication
- PMID: 24899205
- PMCID: PMC4136276
- DOI: 10.1128/JVI.00873-14
Kaposi's sarcoma-associated herpesvirus K3 and K5 ubiquitin E3 ligases have stage-specific immune evasion roles during lytic replication
Abstract
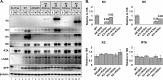
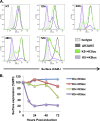
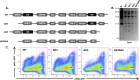
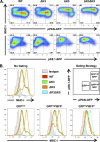
The downregulation of immune synapse components such as major histocompatibility complex class I (MHC-I) and ICAM-1 is a common viral immune evasion strategy that protects infected cells from targeted elimination by cytolytic effector functions of the immune system. Kaposi's sarcoma-associated herpesvirus (KSHV) encodes two membrane-bound ubiquitin E3 ligases, called K3 and K5, which share the ability to induce internalization and degradation of MHC-I molecules. Although individual functions of K3 and K5 outside the viral genome are well characterized, their roles during the KSHV life cycle are still unclear. In this study, we individually introduced the amino acid-coding sequences of K3 or K5 into a ΔK3 ΔK5 recombinant virus, at either original or interchanged genomic positions. Recombinants harboring coding sequences within the K5 locus showed higher K3 and K5 protein expression levels and more rapid surface receptor downregulation than cognate recombinants in which coding sequences were introduced into the K3 locus. To identify infected cells undergoing K3-mediated downregulation of MHC-I, we employed a novel reporter virus, called red-green-blue-BAC16 (RGB-BAC16), which was engineered to harbor three fluorescent protein expression cassettes: EF1α-monomeric red fluorescent protein 1 (mRFP1), polyadenylated nuclear RNA promoter (pPAN)-enhanced green fluorescent protein (EGFP), and pK8.1-monomeric blue fluorescent protein (tagBFP), marking latent, immediate early, and late viral gene expression, respectively. Analysis of RGB-derived K3 and K5 deletion mutants showed that while the K5-mediated downregulation of MHC-I was concomitant with pPAN induction, the reduction of MHC-I surface expression by K3 was evident in cells that were enriched for pPAN-driven EGFP(high) and pK8.1-driven blue fluorescent protein-positive (BFP(+)) populations. These data support the notion that immunoreceptor downregulation occurs by a sequential process wherein K5 is critical during the immediately early phase and K3 plays a significant role during later stages.
Importance: Although the roles of K3 and K5 outside the viral genome are well characterized, the function of these proteins in the context of the KSHV life cycle has remained unclear, particularly in the case of K3. This study examined the relative contributions of K3 and K5 to the downregulation of MHC-I during the lytic replication of KSHV. We show that while K5 acts immediately upon entry into the lytic phase, K3-mediated downregulation of MHC-I was evident during later stages of lytic replication. The identification of distinctly timed K3 and K5 activities significantly advances our understanding of KSHV-mediated immune evasion. Crucial to this study was the development of a novel recombinant KSHV, called RGB-BAC16, which facilitated the delineation of stage-specific phenotypes.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Kaposi's sarcoma-associated herpesvirus ubiquitin ligases downregulate cell surface expression of l-selectin.J Gen Virol. 2021 Nov;102(11). doi: 10.1099/jgv.0.001678. J Gen Virol. 2021. PMID: 34726593
-
Construction and manipulation of a new Kaposi's sarcoma-associated herpesvirus bacterial artificial chromosome clone.J Virol. 2012 Sep;86(18):9708-20. doi: 10.1128/JVI.01019-12. Epub 2012 Jun 27. J Virol. 2012. PMID: 22740391 Free PMC article.
-
Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins.J Virol. 2000 Jun;74(11):5300-9. doi: 10.1128/jvi.74.11.5300-5309.2000. J Virol. 2000. PMID: 10799607 Free PMC article.
-
Immune evasion by a novel family of viral PHD/LAP-finger proteins of gamma-2 herpesviruses and poxviruses.Virus Res. 2002 Sep;88(1-2):55-69. doi: 10.1016/s0168-1702(02)00120-x. Virus Res. 2002. PMID: 12297327 Review.
-
The trafficking and regulation of membrane receptors by the RING-CH ubiquitin E3 ligases.Exp Cell Res. 2009 May 15;315(9):1593-600. doi: 10.1016/j.yexcr.2008.10.026. Epub 2008 Nov 5. Exp Cell Res. 2009. PMID: 19013150 Review.
Cited by
-
Fine-Tuning of the Kaposi's Sarcoma-Associated Herpesvirus Life Cycle in Neighboring Cells through the RTA-JAG1-Notch Pathway.PLoS Pathog. 2016 Oct 19;12(10):e1005900. doi: 10.1371/journal.ppat.1005900. eCollection 2016 Oct. PLoS Pathog. 2016. PMID: 27760204 Free PMC article.
-
Molecular Biology of KSHV in Relation to HIV/AIDS-Associated Oncogenesis.Cancer Treat Res. 2019;177:23-62. doi: 10.1007/978-3-030-03502-0_2. Cancer Treat Res. 2019. PMID: 30523620 Free PMC article.
-
Methylation of KSHV vCyclin by PRMT5 contributes to cell cycle progression and cell proliferation.PLoS Pathog. 2024 Sep 10;20(9):e1012535. doi: 10.1371/journal.ppat.1012535. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39255317 Free PMC article.
-
DExD/H Box Helicases DDX24 and DDX49 Inhibit Reactivation of Kaposi's Sarcoma Associated Herpesvirus by Interacting with Viral mRNAs.Viruses. 2022 Sep 20;14(10):2083. doi: 10.3390/v14102083. Viruses. 2022. PMID: 36298642 Free PMC article.
-
METTL16 controls Kaposi's sarcoma-associated herpesvirus replication by regulating S-adenosylmethionine cycle.Cell Death Dis. 2023 Sep 6;14(9):591. doi: 10.1038/s41419-023-06121-3. Cell Death Dis. 2023. PMID: 37673880 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- AI073099/AI/NIAID NIH HHS/United States
- R01 CA082057/CA/NCI NIH HHS/United States
- CA31363/CA/NCI NIH HHS/United States
- R01 AI073099/AI/NIAID NIH HHS/United States
- R01 CA031363/CA/NCI NIH HHS/United States
- CA180779/CA/NCI NIH HHS/United States
- R01 CA124332/CA/NCI NIH HHS/United States
- K20815000001/PHS HHS/United States
- R01 DE023926/DE/NIDCR NIH HHS/United States
- P01 CA180779/CA/NCI NIH HHS/United States
- DE023926/DE/NIDCR NIH HHS/United States
- R01 CA115284/CA/NCI NIH HHS/United States
- R01 CA177377/CA/NCI NIH HHS/United States
- CA082057/CA/NCI NIH HHS/United States
- CA115284/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

